The Use of Biologic Agents in the Geriatric Population
The aging process is difficult, involving multisystem functional decline, and the pharmacokinetics of medications are altered in older persons, resulting in increased risks. Concerns are demonstrated in case reports.
ABSTRACT: The aging process is difficult, involving multisystem functional decline, and the pharmacokinetics of medications are altered in the older population, resulting in increased risks. Concerns associated with biologic therapy are demonstrated in case reports. Analysis of the literature about biologic therapy for rheumatologic conditions in the geriatric population, such as rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, is limited by a lack of data about older subjects in clinical trials. Safety concerns in older patients include infection, hepatitis B, and infusion reaction risks. For some older patients, biologic therapy is indicated, given the lack of effectiveness of previous therapies, and it provides humane treatment. However, the threshold is much higher in this population than in adults of younger age because of the increased risks. (J Musculoskel Med. 2010;27:175-180)
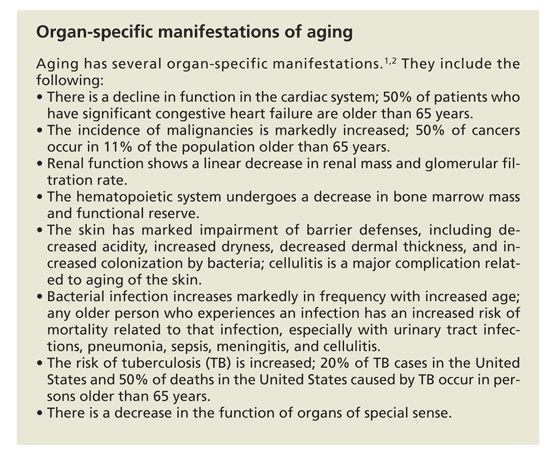
Actress Bette Davis said, “Aging ain’t for sissies.” The aging process is difficult, involving multisystem functional decline in which defects in multiple homeostatic mechanisms are acquired, resulting in a markedly reduced capacity to respond to stress. Although the process is highly time-dependent and mirrors chronological age, in any given person it can be highly variable.1,2 (For more on difficulties in aging, see the Box, “Organ-specific manifestations of aging.”)
In addition, the pharmacokinetics of medications are altered in the older population, resulting in increased risks with their use. As the geriatric population continues to grow, the need for physicians to become more familiar with the special considerations related to medication use in these patients is increasing accordingly.
A number of specific concerns are associated with biologic therapy for older patients. In discussing its use in the geriatric population, several aspects must be considered before an analysis of data that describe outcomes is helpful.
In this article, I discuss the treatment of older patients who have rheumatoid arthritis (RA) and other rheumatologic conditions with biologic agents, particularly the anti–tumor necrosis factor α (anti–TNF-α) therapies. A brief description of 3 actual patients who exemplify some specific concerns will be described, followed by a discussion of the physiology of aging, as well as the effects of aging on the immune system. A review of the literature documenting the outcomes of biologic therapy in older patients with various diseases will be described.
The cases
Special concerns in the use of biologic therapy in older patients are demonstrated in the following 3 patient cases:
Case 1. An 89-year-old woman with psoriatic arthritis (PsA) was referred for further therapy. Her past medical history was notable for a lack of previous significant infections. At age 87 years, 2 years before presentation, she received methotrexate (MTX) and adalimumab for 18 months; there was some improvement of her synovitis.
The patient then was admitted to the medical ICU in acute respiratory distress. All medications were discontinued. She had a prolonged course in the ICU, requiring intubation. Her blood cultures were positive for Escherichia coli. She survived and then was referred to me. This case demonstrates life-threatening infection as a major concern when biologic agents are used in older patients.
Case 2. A 74-year-old woman with a long-standing history of severe RA who had been treated with multiple disease-modifying antirheumatic drugs (DMARDs) was referred for further therapy. Her medical history included a personal history of breast cancer in the distant past. She also had an approximately 40-pack-per-year smoking history, although she had quit smoking cigarettes 20 years before her presentation.
At age 73 years, this patient received etanercept weekly for 6 months. Before this therapy, she had a normal baseline chest x-ray film and negative purified protein derivative test results. Etanercept was discontinued after 6 months because of a lack of effectiveness and the development of a diffuse rash. Five months after etanercept was discontinued, the patient experienced weight loss, a cough, and night sweats and was referred to me for further therapy. A chest x-ray film revealed a new lung mass, the biopsy of which showed adenocarcinoma of the lung. This case demonstrates another important problem of biologic therapy in older patients, an increased risk of malignancy.
Case 3. A 74-year-old woman with severe RA and interstitial lung disease (ILD) was treated with azathioprine (AZA) for several years; her synovitis and pneumonitis were controlled. Four years earlier, she had experienced increasing shortness of breath and a new mass was seen on her chest x-ray film. Biopsy of this mass revealed a low-grade B-cell lymphoma (mucosa-associated lymphoid tissue).
The oncology department treated the patient with rituximab, 375 mg/m2 weekly for 4 weeks, then monthly for 2 years. She then received maintenance therapy with rituximab on a monthly basis; remission of the lymphoma was achieved, as well as complete remission of her rheumatoid synovitis and ILD.
After 2 years of rituximab maintenance, the patient’s treatment was discontinued. She was then referred back to the rheumatology clinic, where her synovitis continues to be in remission but her ILD has flared. Therapy with AZA has been restarted and good control of her pulmonary disease has been achieved. This case demonstrates the potential that rituximab holds for management of rheumatologic diseases in older patients.
The physiology of aging
In addition to the multisystem functional decline that occurs with aging, numerous studies have documented many changes in the immune system. Naylor and associates3 described the influence of age on T-cell generation and T-cell receptor diversity. Thymic output was noted to be markedly decreased by age 65 years. T-cell diversity and the T-cell pool are drastically contracted by age 70 years, and there is a marked inability to mount a T-cell response to new antigens.
Goronzy and colleagues4 described marked telomere loss and decreased length in T cells increasing with age. They also noted that clonal expansion of B cells and T cells is limited, leading to defective immune responses. The authors theorized that these changes lead to a markedly increased risk of infection, increased susceptibility to tumors, and an increased risk of autoimmunity in the older population.
Altered pharmacokinetics
Older age is associated with marked alterations in drug absorption, distribution, activation, metabolism, and clearance. These alterations are related to decreases in lean body mass, total body water, serum albumin level, and renal and hepatic function, as well as an increase in total body fat.1,2
Therefore, in older patients, medications pose an increased risk of myelosuppression, cardiotoxicity, renal insufficiency, and neurotoxicity, as well as the unique effect of polypharmacy on the aging mental status. For these reasons, biologic therapies in which the metabolism is largely unknown but seems to be unrelated to hepatic metabolism or renal excretion (except for anakinra, which has a significant excretion by the kidney) would seem ideal for use in the geriatric population because intrinsically they pose less risk in terms of their routes of elimination.
A limitation to consider when analyzing the literature about biologic therapy in the geriatric population is a lack of data about older subjects in clinical trials.2 Theories explaining this lack include the following1:
• There is an increase in comorbid diseases and conditions with aging.
• A focus on aggressive therapy may be unacceptable to older patients.
• Expectations are limited for long-term treatment benefits in patients for whom the long term is not particularly long.
• Financial, social, and logistical support to assist older patients in trial participation is limited.
As a result, older patients were not specifically recruited into the clinical trials of biologic therapy described here, requiring extrapolation to subgroups of older populations. These data refer predominantly to RA, although some studies include patients with PsA and ankylosing spondylitis (AS). The data also refer predominantly to anti–TNF-α therapy, specifically etanercept, infliximab, and adalimumab. There are no data for abatacept in older populations. There is no actual study of rituximab in RA, but there are abundant data analyzing the use of rituximab in lymphoma for older patients.
Increased risk of infection in RA
Fleischmann and Iqbal5 studied the risk-benefit profile of etanercept in older patients with RA, AS, and PsA. The study involved 3893 patients; 597 (15.3%) were older than 65 years.
The authors found no difference in the efficacy of etanercept in the older patients. There was an absence of demyelination as an adverse effect in older patients. No unique adverse effects were seen in these patients. However, there was a marked increased incidence of adverse effects and serious adverse effects in older patients, especially serious infections, and there were trends for an increased incidence of malignancy and adverse cardiovascular events. Because the small numbers involved and the high dropout rate in these groups compromised the data, the authors could not report a statistically significant difference for these complications compared with the younger cohorts.
Bongartz and associates6 analyzed 5014 patients with RA in 9 trials (3493 of the patients were treated with infliximab or adalimumab and 1512 of the patients were treated with placebo and MTX). No breakdown of adverse effects was reported on the basis of age. However, a pooled odds ratio (OR) of 3.3 for malignancy was reported that excluded the malignancies of lymphoma and skin cancer. In addition, a pooled OR of 2.0 for serious infection was observed (defined as any infection requiring antibiotics or hospitalization or both). A dose-dependent increase in malignancy risk was observed in patients with RA treated with anti–TNF-α therapy.
These data are in contrast to those reported by Schneeweiss and colleagues7 in a cohort study of 15,597 patients with RA 65 years or older (mean age, 76.5 years) who received DMARD therapy. Of these patients, 10,617 received corticosteroids; 1900, MTX; 1957, noncytotoxic DMARDs; 654, cytotoxic DMARDs; and 469 anti–TNF-α therapy.
The outcome was any infection that required hospitalization (defined as serious bacterial infection). Any patient who had an infection and was not hospitalized, received antibiotics at home, or was treated in the emergency department for 1 day or who died without being hospitalized was not counted. In addition, the specific infections of cellulitis, bronchitis, sinusitis, and diverticular abscess, as well as urinary tract infection, were excluded from the definition of serious bacterial infection. Thus, the total number of infections in this older population was markedly underestimated; also, the average follow-up was short.
A rate of serious bacterial infection of 2.2 cases per 100 person-years was observed, revealing no increase in initiates of anti–TNF-α therapy versus other DMARDs or compared with MTX. There was no increase in dose-dependent risk in corticosteroid users compared with MTX users; the relative risk was 1.34 for persons receiving prednisone, 5 mg/d or less, and 5.48 for those receiving prednisone, 20 mg/d or more. In addition, 11 opportunistic infections were reported. Because no data were given about which treatment groups patients were enrolled in, whether the infections were seen predominantly in the patients receiving anti–TNF-α therapy is unknown.
Maillard and associates8 reported a regional cohort study made up of 83 patients with RA or AS that retrospectively analyzed the use of infliximab, 3 mg/kg, over 8-week infusion intervals. A serious pyogenic infection developed in 5 of the patients (mean age, 65.8 years). This finding was statistically significant. All of these patients had RA. The mean corticosteroid dose in this group was 15.5 mg/d versus 6.9 mg/d (also statistically significant). The median time to the diagnosis of the serious pyogenic infection after the start of infliximab therapy was 2 months. Therefore, older age and higher corticosteroid dose were associated with an increased risk of serious pyogenic infection during infliximab treatment.
Takeuchi and coworkers9 conducted a postmarketing study of patients with RA receiving infliximab prospectively monitored for all adverse events for 6 months. There was an increased risk of bacterial infection that correlated with older age, male sex, advanced RA, and chronic obstructive pulmonary disease (COPD). There also was an increased incidence of tuberculosis (TB) and Pneumocystis jiroveci pneumonia, usually occurring within the first month of therapy; the mean age of patients with TB was 66.1 years.
Increased infection risk in other conditions
Patients with giant cell arteritis (GCA) or polymyalgia rheumatica (PMR) are ideal study groups from which to draw conclusions about biologic therapy in older patients because these diseases are seen exclusively in the geriatric population. Hoffman and associates10 reported the outcome of the use of infliximab for maintenance of corticosteroid-induced remission of GCA in a randomized controlled clinical trial at 22 sites with 44 patients who had newly diagnosed GCA. Sixteen patients (median age, 69.5 years) received a corticosteroid plus placebo; 28 patients (median age, 71.5 years) received a corticosteroid and infliximab, 5 mg/kg.
The trial was ended early at 22 weeks for an interim analysis, which revealed no decrease in the relapse rate or ability to taper prednisone to 10 mg/d without relapse. Therefore, infliximab was of no benefit as maintenance therapy. In addition, the use of infliximab may have been harmful-a 71% incidence of infection was seen in the infliximab group (4 serious infections) compared with a 56% incidence in the placebo group (only 1 serious infection). However, these small numbers lacked statistical significance.
Salvarani and colleagues11 offered a companion piece that described infliximab plus prednisone or placebo plus prednisone for the initial treatment of 51 patients with newly diagnosed PMR. The patients were randomized to receive prednisone, 15 mg/d, plus infliximab, 3 mg/kg (median age, 70.9 years) or prednisone, 15 mg/d, plus an infused placebo (mean age, 70.7 years). In both groups, prednisone was tapered to 0 over 16 weeks.
No difference in PMR relapse or recurrence rate was seen between the groups at 1 year. Therefore, infliximab was of no benefit in PMR. It may have been harmful; 2 infections and 4 infusion reactions were seen in the infliximab group and none in the placebo group. However, the study was compromised by a small sample and short follow-up, the low dose of infliximab infused, and a much more rapid corticosteroid taper than generally is used.
Catanoso and colleagues12 reported on the use of etanercept in patients with PMR in a small trial (6 patients, all with relapsed PMR; average age, 75 years). No patient was able to taper prednisone below 7.5 to 10 mg/d because of recurrence of symptoms. The patients received etanercept, 25 mg twice a week SC, for 24 weeks; they were monitored for 9 months.
All 6 patients improved by European League Against Rheumatism criteria, 4 by 70% and 2 by 50%. At 9 months, there was a mean reduction in the median prednisone dosage to 2.5 mg/d versus 8.75 mg/d; this was statistically significant. However, serious infections developed in 3 patients, 2 bacterial and 1 influenza. Thus, the study results suggested that etanercept may have efficacy as a corticosteroid-sparing agent in patients with refractory PMR. However, the study was compromised by its very small sample size and its 50% rate of infection.
Malignancy risk
Another major complication with the use of anti–TNF-α therapy, malignancy, was best described by Stone and colleagues13 reporting for the Wegener’s Granulomatosis Etanercept Trial, a prospective placebo-controlled study of etanercept given in addition to standard therapy for the remission or induction of Wegener granulomatosis (WG). The trial included 180 patients with active WG who were monitored for 27 months.
The main findings were a lack of efficacy of etanercept in the management of WG and a statistically significant increase in malignancies, which consisted of solid tumors that occurred both during and after the study. All patients in the etanercept group with malignancies were older. Cancer developed in 6 patients during the study (average age, 61 years; range, 51 to 73 years) and in 3 patients after the study ended (average age, 62.3 years; range, 43 to 73 years).
No difference was seen between the etanercept and placebo groups in disease severity, sex, personal or family history of cancer, cyclophosphamide use, or cumulative cyclophosphamide dose. Thus, a higher risk of solid tumors with etanercept use was seen in the older group compared with patients who received standard care, that is, cyclophosphamide alone.13
Rennard and coworkers14 conducted another study that provided data documenting an increased malignancy risk with anti–TNF-α therapy in older patients. Because COPD is a progressive smoking- related inflammatory lung disease in which TNF is overexpressed, the authors sought to determine whether infliximab might be an effective and safe therapy for patients with COPD. It was a multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-finding study of 234 patients with moderate to severe COPD; there were 44 weeks of follow-up. Seventy-eight patients received infliximab, 3 mg/kg; 79 patients received infliximab, 5 mg/kg; and 77 patients received placebo infusion. Patients who had a history of malignancy or a history of TB were excluded.
The major findings included a lack of benefit for COPD overall and an increased incidence of pneumonia in both infliximab groups. Most important, malignancies were identified in 9 of 157 patients who received infliximab compared with 1 of 77 placebo-controlled patients. The patients with a diagnosis of malignancy were in the older subgroup (average age, 66.2 years). Five of the 9 cancers presented within the first 24 weeks of the start of infliximab therapy, and 4 additional cancers presented in the 24- to 44-week follow-up period. The 5 patients in whom cancers developed during the study were current smokers (average pack-years, 57.3), and the 4 patients in whom cancers developed in the follow-up period were ex-smokers (average pack-years, 63.6).
All cancers observed in this COPD study occurred in older patients. It appeared that infliximab accelerated the clinical expression of cancers for which older patients had an increased risk.
Rituximab use in older patients
There are no data specific for rituximab for RA in older patients. However, because rituximab use was pioneered by oncologists in non-Hodgkin lymphoma (NHL)-a disease of older persons-safety and efficacy data may be extrapolated from this comparable study group.
McLaughlin and associates15 studied 151 patients with relapsed lymphoma (median age, 58 years; 67 patients were older than 60 years) at 31 centers. The median follow-up was 11.8 months. All patients entering the study had been treated previously with combination chemotherapy, radiation therapy, or bone marrow transplant. All had resistant or relapsed disease, and all therefore were profoundly immunosuppressed before enrollment in the study. All patients were treated with rituximab, 375 mg/m2 weekly with 4 doses, and did not receive corticosteroids during the infusion.
The results included adverse effects; 84% of the patients had a reaction to the first infusion, although all reactions were deemed mild (fevers, chills, headaches, nausea, vomiting, pruritus, and angioedema). Late adverse effects (from day 31 to 1 year)-mild neutropenia, leukopenia, and pure red cell aplasia-were seen in 45 patients. Of 68 infections seen, 61 were deemed “minor,” described as upper respiratory tract infections, urinary tract infections, gastroenteritis, localized herpes simplex, and localized herpes zoster. However, 3 patients were reported to have bacteremia. A response rate of 50% of the NHL also was noted. Thus, rituximab was well tolerated, especially in older patients, and was effective for refractory NHL.
Hainsworth and coworkers16 described 60 patients (median age, 65 years) who had no previous treatment for indolent NHL. All received rituximab, 375 mg/m2, for 4 weeks. All responders then received rituximab maintenance therapy, consisting of 4 doses at 6-month intervals; patients were monitored for 34 months.
Adverse effects, all related to the first infusion, consisted of fever, chills, nausea, and chest pain; all were deemed mild. There was no greater incidence of toxicity in the subgroup of patients older than 70 years within this group, and no opportunistic infections were seen. However, the study did not mention other infections. There also was a 47% response of the lymphoma at 6 weeks and a 73% response after maintenance therapy, in which 37% of patients entered complete remission. Thus, rituximab was not only highly effective first-line therapy for patients with NHL but also extremely safe

and well tolerated, even in patients older than 70 years. The lymphoma data, for which there are now more than 15 years of long-term follow-up, suggest that rituximab may be considered a potentially safer biologic therapy alternative for older patients with RA.
Conclusions
Which older patients should be considered for biologic therapy? The data clearly demonstrate that the ideal patient should have several specific characteristics. See the Box, “The ideal older candidate for biologic therapy.”
Important safety concerns in the older patient population include infection; malignancy, especially solid tumors; and infusion reactions. Physicians need to individualize and stratify on the basis of these risk factors. For a small number of older patients, biologic therapy is indeed indicated, given the lack of effectiveness of previous therapies, and it provides humane treatment. However, the threshold is much higher in the geriatric population than in adults of average age because there is a higher incidence of infection and malignancy in older patients. Thus, primary care physicians should keep these increased risks in mind when referring older patients for biologic therapy.
References:
References1. Cobbs EL, Duthie EH, Murphy JB, eds. Geriatrics Review Syllabus: A Core Curriculum in Geriatric Medicine. 4th ed. New York: Kendall Hunt Publishing Company; 2001:10-21, 202, 233-240, 279-284.
2. Cassel CK, Cohen HJ, Larsen EB, et al, eds. Geriatric Medicine. 3rd ed. New York: Springer Verlag; 1997:3-4, 233-234, 357-359, 586-589, 600-622.
3. Naylor K, Li G, Vallejo AN, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446-7452.
4. Goronzy JJ, Fujii H, Weyand CM. Telomeres, immune aging and autoimmunity. Exp Gerontol. 2006;41:246-251.
5. Fleischmann R, Iqbal I. Risk:benefit profile of etanercept in elderly patients with rheumatoid arthritis, ankylosing spondylitis or psoriatic arthritis. Drugs Aging. 2007;24:239-254.
6. Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275-2285.
7. Schneeweiss S, Setoguchi S, Weinblatt ME, et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:1754-1764.
8. Maillard H, Ornetti P, Grimault L, et al. Severe pyogenic infections in patients taking infliximab: a regional cohort study. Joint Bone Spine. 2005;72:330-334.
9. Takeuchi T, Tatsuki Y, Nogami Y, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:189-194.
10. Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis. Ann Intern Med. 2007;146:621-630.
11. Salvarani C, Macchioni P, Manzini C, et al. Infliximab plus prednisone or placebo plus prednisone for the initial treatment of polymyalgia rheumatica: a randomized trial. Ann Intern Med. 2007;146:631-639.
12. Catanoso MG, Macchioni P, Boiardi L, et al. Treatment of refractory polymyalgia rheumatica with etanercept: an open pilot study. Arthritis Rheum. 2007;57:1514-1519.
13. Stone JH, Holbrook JT, Marriott MA, et al; Wegener’s Granulomatosis Etanercept Trial Research Group. Solid malignancies among patients in the Wegener’s Granulomatosis Etanercept Trial. Arthritis Rheum. 2006;54:1608-1618.
14. Rennard SI, Fogarty C, Kelsen S, et al; COPD Investigators. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:926-934.
15. McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825-2833.
16. Hainsworth JD, Litchy S, Burris HA 3rd, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-hodgkin’s lymphoma. J Clin Oncol. 2002;20:4261-4267.