All about osteoporosis: A comprehensive analysis
Osteoporosis is a major public health threat that affects more than half of persons older than 50 years. The most common bone disease in humans, osteoporosis is associated with pain, disability, and increased risk of mortality.
Osteoporosis is a major public health threat that affects more than half of persons older than 50 years. The most common bone disease in humans, osteoporosis is associated with pain, disability, and increased risk of mortality.
The primary reference that clinicians use in managing osteoporosis is the National Osteoporosis Foundation’s (NOF) Clinician’s Guide to Prevention and Treatment of Osteoporosis, published in 1999 and updated in 2003. The guidelines in this publication represented the standard of care but did not adequately address some populations that may be affected by osteoporosis. For example, little guidance was offered on how to treat men with osteoporosis or how to approach the variations associated with ethnicity.
Many clinicians welcomed the comprehensive revised NOF guidelines released in February 2008, appreciating the new practice parameters that addressed previous areas of concern not mentioned in the 2003 document. The NOF guidelines were complemented by release of the World Health Organization’s (WHO) fracture risk assessment tool (FRAX), which helped physicians and patients gain a better understanding of a specific patient’s 10-year absolute risk of an osteoporosis-related fragility fracture.
The primary goal of treatment is to reduce the incidence and the morbidity and mortality associated with osteoporosis-related fragility fractures. Treatment often includes lifestyle modifications, such as performance of weight-bearing exercises.
A number of pharmacological therapies are currently available, although gaining patients’ adherence to therapy remains a challenge. Several new agents are under investigation.
This 2-part article provides an overview of osteoporosis diagnosis and management. In this first part, we interpret and summarize recent publications in this field; pool the data that are of greatest relevance to primary care physicians, including the epidemiology; review technologies available for diagnosis; and help decipher whom to test and to treat. The second part, to appear in a later issue of this journal, will discuss the current therapies for osteoporosis, their adverse effects, and reasonable approaches to monitoring.
DIAGNOSIS
Epidemiology
Osteoporosis is a threat to an estimated 44 million Americans. Ten million Americans are thought to already have the disorder and an estimated 34 million more to have low bone mass.1
Significant risk has been reported in all ethnicities. An estimated 20% of white and Asian women older than 50 years are thought to have osteoporosis; in a similar age-group, 5% of African American women and 10% of Hispanic women are thought to have the disease.
Osteoporosis risk is increasing most rapidly among Hispanic women.1 Some studies have shown that they consume less calcium than the recommended dietary allowance in all age-groups. Also, Hispanic women are twice as likely as white women to have diabetes mellitus, which may increase their risk of osteoporosis.2 Although osteoporosis is largely thought of as a disease that affects women, its prevalence is 7% in white men, 5% in African American men, and 3% in Hispanic men.3
Research into the diagnosis and management of osteoporosis continues to expand; the disease is projected to affect an even larger portion of the US population over the next 15 years as the average age increases. The economic burden is estimated to rise to more than $17 billion annually in the United States alone.4 By 2025, the costs of osteoporotic-related fractures are expected to rise to about $25 billion.1 The economic consequence is proposed to increase by about 50%.
A dramatic reality is that 1 of 2 women and 1 of 4 men 50 years and older probably will have an osteoporosis-related fracture in their lifetime. Therefore, clinicians should be well aware of osteoporosis and have a good understanding of its diagnosis and appropriate management.
Definition
The NOF defines osteoporosis as a disease characterized by low bone mass and structural deterioration of bone tissue that lead to bone fragility and an increased susceptibility to fractures
(especially of the hip, spine, and wrist, although any bone can be affected).1 The WHO defines osteoporosis as a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fractures.5
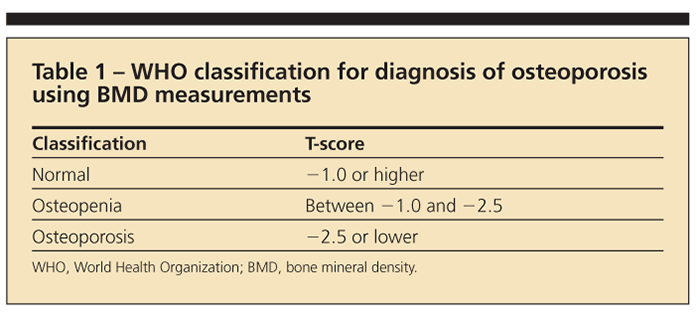
The WHO further defines osteoporosis through radiographic parameters obtained from dual-energy x-ray absorptiometry (DXA) on the basis of its 1994 classification (Table 1).5 T-score refers to the standard deviation between a patient’s bone mineral density (BMD) and that of a young adult in the reference population. The Z-score is a comparison of the patient’s BMD with an age-matched population.5
Osteopenia is now generally accepted as being synonymous with low bone mass. Note that patients with low bone mass are not necessarily at high risk for fracture.6
A diagnosis of osteoporosis may be made clinically by the occurrence of fragility fractures. In their absence, however, the gold standard test for low BMD and osteoporosis is DXA. A fragility fracture is defined as a pathological fracture that occurs as a result of a fall from a standing height or lower. Fractures in this context suggest weakness of the bony skeleton.
However, bone strength reflects not only BMD-the amount of calcium hydroxyapatite per square centimeter of bone-but also integration of bone density with bone quality (the architecture, turnover, damage accumulation [eg, microfractures], and mineralization of bone).7 Despite widespread use of DXA for measurement of BMD, currently there is no simplistic way to measure bone quality that also can affect overall bone strength.
Risk factors
Clinical experience has highlighted a number of risk factors related to the development of osteoporosis and fragility fractures (Table 2). Some are modifiable, but others, such as genetic and ethnic predisposition, are beyond the control of the patient and clinician.
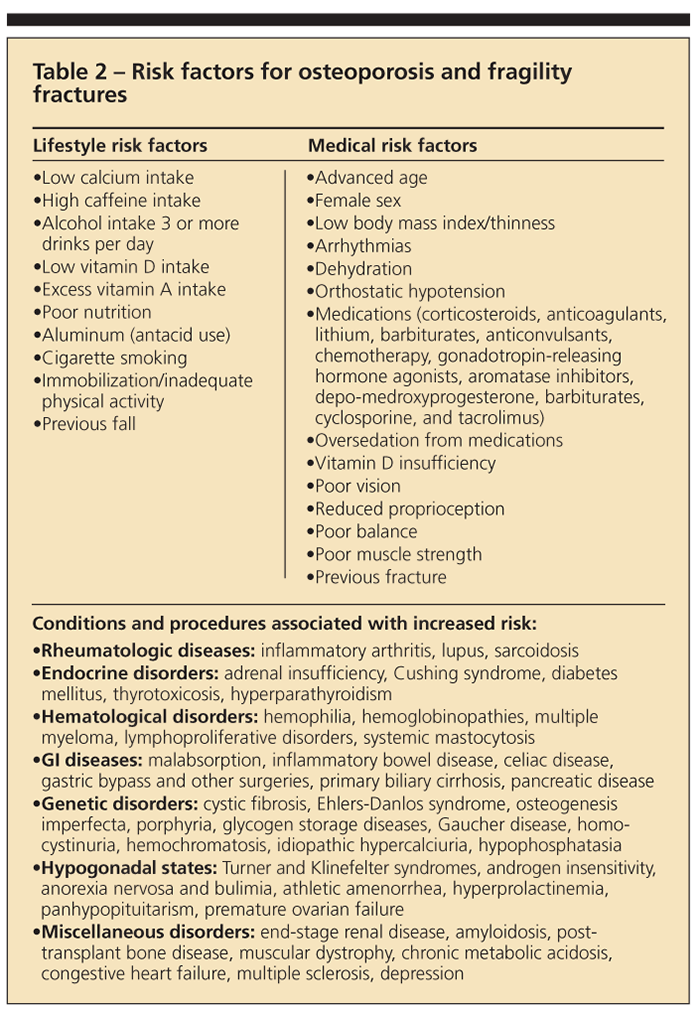
Fluctuating hormone levels in both men and women are known risk factors. Low estrogen levels in women (menopause and amenorrhea) and low levels of testosterone in men are both implicated. There is an increased risk with older age, a history of previous fracture, and a family history of osteoporosis or family history of previous fracture. Other reported variables that influence osteoporosis development include low body mass index; inactivity; cigarette smoking; excessive alcohol intake (defined as 3 or more drinks per day); and a diet low in calcium and vitamin D but high in protein, sodium, and caffeine.
The use of a number of medications has been associated with an increased risk of osteoporosis. Systemic corticosteroids, heparin, levothyroxine replacement therapy, and anticonvulsant therapy are among the most common.1 Diseases that may result in an increased risk of secondary osteoporosis include endocrinopathies, malabsorptive GI conditions, congestive heart failure, blood dyscrasia, osteogenesis imperfecta, amyloidosis, and inflammatory arthropathies.1
Available diagnostic technology
Factors that have made DXA the most widely used imaging technique for osteoporosis include low-dose radiation, availability, capacity to evaluate multiple sites, and ease of use.8 DXA also is the most widely used method for measuring BMD because it provides very precise measurements.
To optimize the accuracy of results and be able to compare studies obtained from DXA imaging over time, each center is required to calculate a precision error (PE) and least significant change (LSC). The PE, the variation in results of repeated measurements in the same group of patients over a short period, may be obtained with a PE calculator on the International Society for Clinical Densitometry Web site (http://www.iscd.org). The LSC is determined by multiplying the PE by 2.8 (95% confidence inter-val).9 Having calculated the LSC, a clinician can determine whether a change in BMD-increase or decrease-is clinically significant and thus indicative of a need for a change in treatment.
Many studies have demonstrated that low BMD evidenced by DXA at any site can predict osteoporotic fracture, although hip measurements are superior to those in the lumbar spine in predicting hip as well as overall osteoporotic fracture risk.10 DXA may be limited in older patients because degenerative changes that may skew the results toward overestimation of BMD are present. Other disadvantages of conventional DXA are that the machine is large and therefore not portable, is expensive, and uses ionizing radiation.9
One study reported that measurement of BMD in the distal forearm and calcaneus with portable densitometers provides valid indicators of BMD in central anatomical sites.11 However, this study was done on preadolescent and adolescent women, a group not representative of the population most affected by osteoporosis. Therefore, the validity of portable DXA remains questionable. Some DXA machines are expected to provide reports that include information on a person’s absolute fracture risk.1
DXA measures bone mass per cross-sectional area (the area density).12 In contrast, quantitative CT (qCT) measures volumetric bone density of the spine and hip and can analyze cortical and trabecular bone separately. qCT currently is not recommended for screening, largely because the application of T-scores to predicting the risk of fracture has not been validated with this modality. In addition, qCT is more costly than DXA and results in greater exposure to radiation.13 qCT methodology is quite useful in clinical research and may be used to follow therapeutic responses in individual patients, particularly in relation to intermittent parathyroid hormone therapy.
An advantage of qCT is that any commercially available CT scanner may be used; the only additional cost to the practice or institution using it results from the acquisition of special software. In addition, volumetric BMD measurements obtained with this modality are independent of the area of the vertebral body so that vertebral size does not cause errors. As a result, there is minimal interference of measurements by degenerative joint disease,14 as well as reduced interference of measurements in patients with fracture or sclerotic or lytic lesions of bone.
Quantitative ultrasonography, another method for measuring bone density, appears to be a good predictor of fractures in men and women and is at least as useful as clinical risk factors for identifying patients at high risk for osteoporosis.10 However, a meta-analysis of 25 studies that evaluated the sensitivity and specificity of calcaneal ultrasonography for identifying patients with DXA T-scores of 22.5 or lower concluded that current- ly used ultrasonography cutoff thresholds do not have sufficiently high sensitivity or specificity to definitively exclude or confirm DXA-diagnosed osteoporosis.
The criteria for making a diagnosis of osteoporosis and recommending treatment on the basis of the results of ultrasonography are not yet well established. In addition, ultrasonography cannot be counted on for reliability in monitoring patients who are treated for osteoporosis because of limited precision and a slow rate of change of bone mass at peripheral sites. Thus, most persons at high risk for an ultrasonographic finding will need a confirmatory DXA both to determine the need for treatment on the basis of well-established guidelines and to serve as a baseline for monitoring therapy.
A cost-effectiveness analysis evaluated the use of quantitative ultrasonography as a prescreening tool for determining which women need DXA evaluation. Although the sensitivity and specificity of ultrasonography were high, such screening was not found to be cost-effective.10
Fewer data are available for bone MRI, and less emphasis has been placed on MRI as a modality for evaluating osteoporosis, largely because of the expense involved. A study that compared qCT with MRI reported that the modalities perform equally well in providing information about trabecular structure.15 MRI has the advantages of being nonionizing and offering multiplanar acquisition, but its use is hampered by high cost and low availability.
Indications for BMD testing
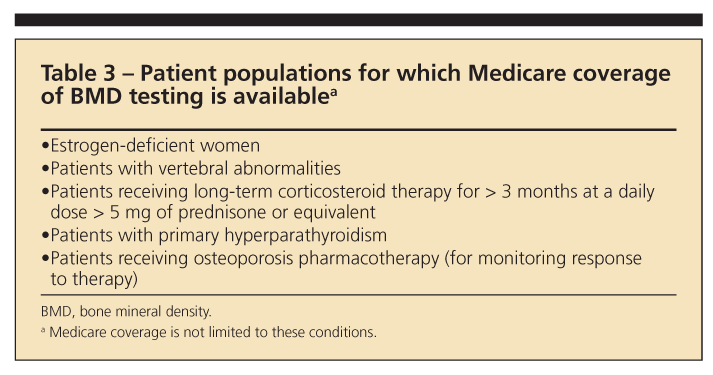
The NOF has provided a number of indications for BMD testing. However, some restraints may prevent testing on the basis of an individual patient’s insurance coverage (Table 3). The recommended indications for testing are the following1:
•Women 65 years and older and men 70 years and older, regardless of clinical risk factors.
•Younger postmenopausal women and men 50 to 69 years about whom the physician has concern based on their clinical risk factor profile.
•Women in the menopausal transition, if there is a specific risk factor associated with increased fracture risk, such as with low body weight, previous low-trauma fracture, or high-risk medication (for example, prednisone).
•Persons who have fracture after age 50 years.
•Adults who have conditions or are taking medications associated with low bone mass or bone loss.
•Any persons who are being considered for pharmacological therapy for osteoporosis.
•Anyone being treated for osteoporosis (to monitor therapy).
•Anyone in whom evidence of bone loss would warrant treatment.
•Postmenopausal women who are discontinuing the use of estrogen.
Fracture risk assessment tool
The introduction of the FRAX score by the WHO broadened the considerations given to the diagnosis and management of patients with low BMD. The FRAX score, which has been incorporated into the NOF’s 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis, integrates BMD measures with clinical risk factors for fragility fractures; with the utility of algorithms, it provides a 10-year probability of hip and other major osteoporotic fracture at various sites, such as the vertebrae, forearm, and shoulder.16 A detailed description of the FRAX algorithm is available online at http://www.nof.org.
SUMMARY
Osteoporosis is a major health care concern associated with significant costs. Guidelines provided by the NOF and the WHO help the diagnosis, management, and monitoring of this disease. DXA is the gold standard imaging technique. Continued research and the development of additional diagnostic and therapeutic modalities are needed.
References:
References1. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008. http://www.orbdnrc.nof.org/professionals/NOF_Clinicians_Guide.pdf. Accessed March 22, 2010.
2. The NIH Osteoporosis and Related Bone Diseases National Resource Center. http://www.niams.nih.gov/bone. Accessed March 17, 2010.
3. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians [published correction appears in Ann Intern Med. 2008;148:888]. Ann Intern Med. 2008;148:680-684.
4. Shah A. Treatment of osteoporosis: a patient-centered approach. The Osteoporosis Report. 2009;1(1). http://www.theosteoporosisreport.com. Accessed March 22, 2010.
5. Leslie WD, Adler RA, El-Hajj Fuleihan G, et al; International Society for Clinical Densitometry. Application of the 1994 WHO classification to populations other than postmenopausal Caucasian women: the 2005 ISCD Official Positions. J Clin Densitom. 2006;9:22-30.
6. Binkley N, Bilezikian JP, Kendler DL, et al; International Society for Clinical Densitometry. Official positions of the International Society for Clinical Densitometry and Executive Summary of the 2005 Position Development Conference. J Clin Densitom. 2006;9:4-14.
7. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:404-415.
8. Rossini M, Viapiana O, Adami S. Instrumental diagnosis of osteoporosis. Aging (Milano). 1998;10:240-248.
9. Deal CL. Osteoporosis: focus on DXA interpretation. American College of Rheumatology Skills Training Workshop Series; 2006.
10. Raisz GR. Screening for osteoporosis. UpToDate for patients. http://www.uptodate.com/online. Accessed March 17, 2010.
11. Hernandez-Prado B, Lanzcano-Ponce E, Cruz-Valdez A. Validity of bone mineral density measurements in distal sites as an indicator of total bone mineral density in a group of pre-adolescent and adolescent women. Arch Med Res. 2002;33:33-39.
12. Ebbesen EN, Thomsen JS, Beck-Nielsen H, et al. Vertebral bone density evaluated by dual-energy x-ray absorptiometry and quantitative computed tomography in vitro. Bone. 1998;23:283-290.
13. Glüer CC, Engelke K, Lang TF, et al. Quantitative computed tomography (QCT) of the lumbar spine and appendicular skeleton. Eur J Radiol. 1995;20:173-178.
14. International Society of Clinical Densitometry. Bone Densitometry Course: Clinician Course Syllabus and Associated Reading Materials. 2008:30-31.
15. Krug R, Carballido-Gamio J, Burghardt A. Assessment of trabecular bone structure comparing magnetic resonance imaging at 3 Tesla with high-resolution peripheral quantitative computed tomography ex vivo and in vivo. Osteoporos Int. 2008;19:653-661.
16. Watts NB, Lewiecki EM, Miller PD, et al. National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom. 2008;11:473-477.