All about osteoporosis: Meeting the treatment challenge
Following the release of revised National Osteoporosis Foundation guidelines and of the World Health Organization’s fracture risk assessment tool, several pharmacological therapies have become available to help improve outcomes in patients with osteoporosis.
In the wake of recent advances in osteoporosis diagnosis-including release of revised National Osteoporosis Foundation guidelines and of the World Health Organization’s fracture risk assessment tool-several pharmacological therapies have become available or are under investigation to help improve patient outcomes. In this 2-part article, we provide an overview of osteoporosis diagnosis and management. The first part (“All about osteoporosis: A comprehensive analysis,” The Journal of Musculoskeletal Medicine, April 2010, page 149) interpreted and summarized recent publications in this field; pooled the data that are of greatest relevance to primary care physicians, including the epidemiology; reviewed technologies available for diagnosis; and helped decipher whom to test and to treat. In this second part, we discuss lifestyle modifications, the current therapies, their adverse effects, and reasonable approaches to monitoring.
Lifestyle modification
Lifestyle changes that are beneficial for patients with osteoporosis include regular performance of weight-bearing exercises, smoking cessation, moderation in alcohol intake, and maintaining a well- balanced diet that includes adequate calcium and vitamin D intake. The latter are used widely. Current recommendations are a total intake of more than 1200 mg/d of calcium and 800 to 1000 IU/d of vitamin D.1 However, the evidence suggests that calcium and vitamin D provide only modest protection against fragility fractures and should be used in association with other drugs. No clinically important adverse events have been noted with their use.2
Approved agents
Antiresorptive and anabolic agents are used to treat patients who have osteoporosis. Antiresorptive drugs slow bone resorption and bone remodeling, resulting in stabilization and improvement in bone mineral density (BMD).3 Some agents do so by promoting osteoclast apoptosis, which limits bone turnover by stabilizing the calcium-phosphorus surface at the resorptive cavity.4
The FDA-approved members of the antiresorptive class include bisphosphonates (alendronate, ibandronate, risedronate, zoledronic acid), calcitonin, estrogen replacement therapies, and
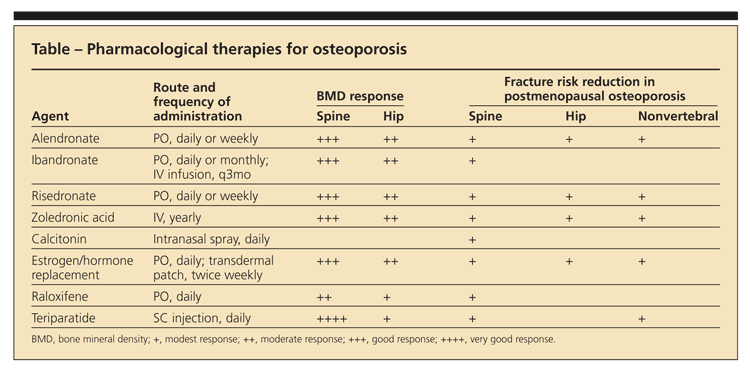
raloxifene (a selective estrogen receptor modulator [SERM]) (Table). The oral bisphosphonates are approved for prevention and management of postmenopausal osteoporosis in women, for osteoporosis in men, and for corticosteroid-induced osteoporosis.
In a 3-year study of postmenopausal women with osteoporosis, vertebral fracture risk was decreased by 70%, hip fracture risk by 41%, and nonvertebral fracture risk by 25% with the use of zoledronic acid.3 Apart from its higher efficacy in decreasing fracture risk compared with oral agents, this drug also is conveniently dosed as a yearly infusion and limits the adverse GI effects associated with oral bisphosphonates.
Ibandronate has been shown to reduce vertebral fractures and is effective in preventing vertebral as well as nonvertebral and hip fractures. Calcitonin is approved for the treatment of women who are at least 5 years postmenopausal, but it has not been shown to decrease the risk of nonvertebral fractures. Hormone therapy is approved for the prevention of fractures in early postmenopausal women. Raloxifene, a SERM agonist in bone, is used for osteoporosis in postmenopausal women; it has the added benefit of reducing the risk of invasive breast cancer.3
Anabolic agents preserve BMD by stimulating the production of bone tissue. Teriparatide, a recombinant form of parathyroid hormone, is the only anabolic agent that has received FDA approval. It is indicated for the management of osteoporosis in postmenopausal women and in men with primary or hypogonadal osteoporosis who are at high risk for fracture. Teriparatide is an alternative for patients who cannot tolerate bisphosphonates; it usually is reserved for patients who have a contraindication to these agents or for whom they are ineffective. The disadvantages of teriparatide include high cost and the need for patients to administer a daily injection.3
Agents in development
A number of osteoporosis agents are in development. Arzoxifene, lasofoxifene, and bazedoxifene are SERMs that are being studied.
Denosumab, a humanized monoclonal antibody against receptor activator of nuclear factor-κB, has been noted to reduce spinal fractures in patients with prostate cancer by 62% compared with placebo.5 Denosumab given subcutaneously twice yearly also is associated with a reduction in the risk of vertebral, nonvertebral, and hip fractures in postmenopausal women with osteoporosis; this agent currently is under review for FDA approval.
Other agents in development include the following:
• Odanacatib is an inhibitor of cathepsin K, which degrades bone and is released by osteoclasts during resorption.
• ZT-031, another parathyroid analogue, may be made available in an inhaled form. A neutralizing antibody to sclerostin-an endogenous osteocyte-derived protein that inhibits bone-stimulating activity-is being studied.
• Strontium is an investigational agent that both reduces the rate of bone resorption and promotes the growth of new bone; it is already being used in Europe.
• Tibolone, a synthetic steroid that is related to progestin, produces metabolites that bind to the estrogen receptors and has been shown to increase BMD. It is currently approved for use in Europe but not in the United States.5
Complications of treatment
Difficulty in gaining long-term adherence to therapy from patients with osteoporosis may adversely affect the effectiveness of treatment. About 50% of patients who have osteoporosis do not take their medications; most discontinue treatment within 1 year.
However, the advent of drugs to be taken once monthly and once yearly may result in improved adherence because there should be less concern for potential adverse effects. A review paper that summarized 15 studies concluded that treatment with oral ibandronate, 150 mg once monthly, is at least as effective as the daily regimen and is associated with increased adherence.6 Another study concluded that both intravenous ibandronate taken monthly and intravenous zoledronic acid taken yearly are associated with improved adherence.7
Among the adverse events that contribute to poor adherence are GI upset and ulcerations and concerns about atrial fibrillation and osteonecrosis of the jaw (ONJ). Factors that increase the risk of ONJ include corticosteroid use, cancer, anticancer therapy, duration of bisphosphonate use, cigarette smoking, preexisting dental disease, and intravenous bisphosphonates.
In 2 clinical trials of zoledronic acid versus placebo for the management of osteoporosis, there were 2 potential cases of ONJ. The risk of ONJ is estimated at about 1 in 10,000 to 1 in 100,000 patient-years in patients taking oral bisphosphonates for osteoporosis.8
Bisphosphonates also are associated with myalgia and arthralgia. The possibility of an increased risk of serious atrial fibrillation with bisphosphonate use was previously of concern when data from a meta-analysis of randomized controlled trials suggested this association.9 However, the FDA later stated that a review of clinical trial data revealed no clear association between overall bisphosphonate exposure and the rate of serious or nonserious atrial fibrillation.
Estrogen and estrogen receptor agonists and antagonists increase the risk of venous thromboembolic events, cerebrovascular accidents, cancer, and gynecological problems.3 The most common short-term adverse effects of teriparatide use are hypercalcemia and hypercalciuria. The most worrisome long-term adverse event is the development of osteosarcoma.3
Monitoring therapy
Monitoring a patient’s treatment progress and determining the appropriate time to terminate therapy are important. The International Society for Clinical Densitometry recommends follow-up BMD testing (dual-energy x-ray absorptiometry [DXA] of the spine and hip) when the expected change in BMD equals or exceeds the least significant change (LSC); this typically occurs 1 or 2 years after the start of or a change in therapy, but the intervals between DXA measurements are longer once therapeutic effect is established.10 When factors that are associated with rapid bone loss (eg, corticosteroid therapy) are present, more frequent testing is appropriate.3
BMD provides evidence of treatment response, as does assessment of biochemical markers. In addition, the latter confirms patient adherence; it typically is reserved for patients who have conditions that might interfere with drug absorption or efficacy, such as malabsorption. In these patients, fasting urinary N-telopeptide (NTX) or serum carboxyterminal collagen crosslinks (CTX) should be measured before and 3 to 6 months after antiresorptive therapy is started. A decrease greater than 50% in urinary NTX excretion or 30% in serum CTX provides evidence of adherence and drug efficacy.10 In contrast, teriparatide increases markers of bone turnover within 3 to 6 months.
Creatinine clearance must be checked at least annually in patients who are receiving bisphosphonates.10 Bisphosphonate use is not recommended for patients who have a creatinine clearance lower than 35 mL/min/1.73m2 because of a lack of clinical experience and supporting data in patients with significant renal impairment.
Suboptimal response to therapy is defined as when a patient has a fracture or a BMD decrease more than the LSC or when bone markers do not decrease at least 50% from baseline levels during antiresorptive therapy. At this time, secondary causes of osteoporosis and a lack of adherence should be investigated as reasons for a lack of treatment success.
There is still no consensus about the ideal time to discontinue therapy. The decision to do so should be based on the patient’s response to therapy and which agent is used.
The Fracture Intervention Trial Long-term Extension (FLEX) studied 1099 postmenopausal women who previously had received alendronate for 5 years in the Fracture Intervention Trial (FIT).11 At the completion of FIT, women were randomly assigned to an additional 5 years of alendronate, 5 or 10 mg/d, or placebo.
The BMD, bone marker, and fracture data in the women who continued alendronate for 10 years were similar to the data in those who did not. In the women who were switched to placebo after 5 years of alendronate therapy, there was a gradual decline in BMD, but mean BMD remained at or higher than levels 10 years earlier. There also was a gradual rise in biochemical markers of bone turnover.
In the placebo groups, the rate of nonvertebral fracture or morphometric vertebral fractures, detected by lateral spine radiographs, was not significantly different from that in the alendronate group. However, there was a slightly higher risk of clinically detected vertebral fracture. There were no differences in adverse events between the groups. The authors concluded that stopping alendronate therapy after 5 years results in a gradual decline in BMD and an increase in biochemical markers of bone turnover but no significantly higher risk of fracture (except for clinical vertebral fracture).
Thus, stopping bisphosphonate therapy after 5 years, with careful BMD and risk factor assessment follow-up, may be reasonable for some women. However, because the FLEX trial did not address the impact of stopping therapy in women at highest risk for fracture, continuing alendronate therapy in these women is recommended for up to 10 years.12
References:
References1. The NIH Osteoporosis and Related Bone Diseases National Resource Center. http://www.niams.nih.gov/bone. Accessed March 17, 2010.
2. Binkley N, Bilezikian JP, Kendler DL, et al; International Society for Clinical Densitometry. Official positions of the International Society for Clinical Densitometry and Executive Summary of the 2005 Position Development Conference. J Clin Densitom. 2006;9:4-14.
3. Cosman F, Dempster DW, Gass ML, et al. Optimizing treatment decisions in osteoporosis: the what, when, and how of treatment. Curr Med Evidence. 2009;2(5).
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008. http://www.nof.org. Accessed March 22, 2010.
5. Cosman F, Dempster DW, Gass ML, et al. Clinical and mechanistic insights into novel agents in development for osteoporosis. Curr Med Evidence. 2009;2(5).
6. Rossini M, Viapiana O, Gatti D, Adami S. Once-monthly oral ibandronate in postmenopausal osteoporosis: translation and updated review. Clin Ther. 2009;31:1497-1510.
7. Boonen S, Vanderschueren D, Venken K, et al. Recent developments in the management of postmenopausal osteoporosis with bisphosphonates: enhanced efficacy by enhanced compliance. J Intern Med. 2008;264:315-332.
8. Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479-1491.
9. Loke YK, Jeevanantham V, Singh S. Bisphosphonates and atrial fibrillation: systematic review and meta-analysis. Drug Saf. 2009;32:219-228.
10. Qiao Z. Monitoring the effects of treatment of osteoporosis-is it worthwhile? The Osteoporosis Report. 2009;1(1):19-22. http://www.theosteoporosisreport.com.
11. Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
12. Rosen HN. Bisphosphonates in the management of osteoporosis in postmenopausal women. UpToDate for patients. http://www.uptodate.com/online. Accessed March 17, 2010.