Epidural Steroid Meningitis Outbreak: CDC Issues New Physician Guidance
Physicians should contact all patients who received potentially contaminated spinal steroid injections after May 21, in light of a multi-state epidemic of meningitis found to be caused by a fungus.
The US Centers for Disease Control and Prevention has now issued detailed guidance for physicians (Interim Treatment Guidance for Central Nervous System and/or Parameningeal Infections Associated with Injection of Potentially Contaminated Steroid Products) in response to an outbreak of meningitis that has spread so far to eight Midwestern and Eastern states, traced to a fungal infection in preservative-free methylprednisolone acetate used for spinal injections.
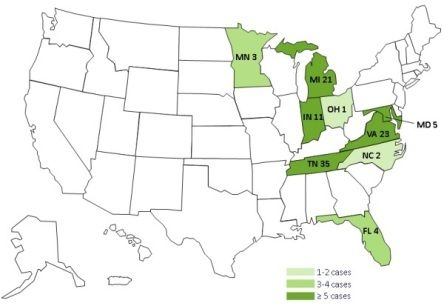
Several patients have had strokes as a result of the meningitis. The CDC announced today that the distributor, New England Compounding Center has voluntarily recalled all products currently in circulation from the Framingham MA facility to which the outbreak has been traced. The product was shipped to 23 states.
Physicians are directed to contact all patients who received medications associated with this product. Red-flag symptoms include fever, new or worsening headache, neck stiffness, sensitivity to light, new weakness or numbness, increasing pain, redness or swelling of the injection site.
The first potentially contaminated injections were given on May 21. The case count is up to 105 thus far, and 8 patients have died.