Psoriatic Arthritis: Expanding Therapeutic Options
The therapeutic options for psoriatic arthritis (PsA) have expanded rapidly and improved patients’ pain, function, and quality of life. However, many patients do not respond to the current disease-modifying medications or cannot take them. Oral methotrexate is the leading disease-modifying antirheumatic drug used in PsA.
ABSTRACT: The therapeutic options for psoriatic arthritis (PsA) have expanded rapidly and improved patients’ pain, function, and quality of life. However, many patients do not respond to the current disease-modifying medications or cannot take them. Oral methotrexate is the leading disease-modifying antirheumatic drug used in PsA. Anti–tumor necrosis factor α (anti–TNF-α) agents have been successful, but many patients do not achieve the American College of Rheumatology 20 end point. Abatacept and rituximab have proved effective for patients with rheumatoid arthritis who have had an inadequate response to anti–TNF-α agents, but data from pilot studies for PsA highlight the different pathophysiological mechanisms underlying the diseases. Few options are available for patients with PsA whose treatment with TNF-α inhibitors is not successful, but the pipeline looks promising. (J Musculoskel Med. 2011;28:163-170)
For patients with psoriatic arthritis (PsA), the rapid expansion of therapeutic options, particularly the anti–tumor necrosis factor α (anti–TNF-α) agents, has contributed to great improvement in pain, function, and quality of life. However, many patients do not respond to the current disease-modifying medications or cannot take these agents because of their cost or adverse effects. In addition, consideration of important metabolic, vascular, and extra-articular comorbidities is essential if physicians are to provide patients with high-quality comprehensive care. Fortunately, the pipeline of potential agents against novel targets for patients with psoriatic disease holds great promise for continued advances in the near future.
In this 2-part article, we provide an update on the diagnosis and management of PsA. The first part (“Psoriatic arthritis: A disease in full” The Journal of Musculoskeletal Medicine, March 2011, page 95) described advances in the assessment of early and established PsA and the efforts under way to develop accurate and reliable biomarkers, as well as the expanding role of imaging studies in diagnosis and assessment of patients with psoriasis and PsA. This second part reviews the latest studies that address the effectiveness of methotrexate (MTX) and biologic agents for patients with psoriatic disease and provides a brief overview of new agents in the pipeline.
CURRENT AGENTS
MTX
Oral MTX is the leading disease-modifying antirheumatic drug (DMARD) used in the treatment of patients with PsA because of its low cost, demonstrated effectiveness in plaque psoriasis and rheumatoid arthritis (RA), and somewhat benign safety profile. Although MTX currently is used in clinical practice, placebo-controlled double-blind trials are urgently needed to examine the efficacy of this agent in MTX-naive populations.
In the Methotrexate in Psoriatic Arthritis (MIPA) trial, 221 patients who had PsA with more than 1 joint involved were enrolled in a 6-month study of MTX versus placebo.1 At the 6-month end point, 71 patients remained in the MTX arm and 77 in the placebo arm. The dose of MTX was set at 15 mg/wk; the primary outcome measure was change in the Psoriatic Arthritis Response Criteria (PsARC).
No difference was noted in the percentages of patients in the treatment and control groups who achieved the PsARC, American College of Rheumatology (ACR) 20 response, or 28-joint Disease Activity Score (DAS28) at 3 or 6 months. However, physician global and skin scores were significantly better in the MTX group. Of note, the number of swollen joints and acute phase reactants (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP] level) did not differ between the groups. Concerns remain about the relatively low dose of MTX, the oligoarticular pattern (median joint count, 5), and the possibility that the study may not have been adequately powered. However, the lack of a decline in swollen joint count or acute phase reactants in the treatment group strongly suggests that MTX is not a disease-modifying agent in PsA.
In the REmicade Study in PsA patients Of methotrexate-Naive Disease (RESPOND) trial, Raffayova and coworkers2 performed a randomized, prospective, multicenter trial to assess the effect of infliximab and MTX compared with that of MTX alone in MTX-naive patients who have active, polyarticular PsA. Active PsA was defined as involvement of 5 or more swollen and tender joints, plus 1 of the following: ESR, 28 mm/h or higher; CRP level, 15 mg/L or higher; and morning stiffness, 45 minutes or longer. Overall, patients had severe disease-mean swollen and tender joint counts of more than 14 and 20, respectively; mean CRP level, higher than 25 mg/dL; and mean Psoriasis Area and Severity Index (PASI) score, higher than 8. The two arms were randomized to infliximab at weeks 0, 2, 6, and 14 plus 15 mg/wk of MTX or to MTX alone. The primary end point was the proportion of ACR 20 improvement at week 16.
As expected, the ACR 20 response with the combination of MTX and infliximab was excellent (86.3%). Also impressive was the response to MTX monotherapy (ACR 20, 66.7%; ACR 50, 39.6%; and ACR 90, 18.8% [all statistically significant]). The rates of DAS28 remission were higher with the combination therapy (68.6%) than with MTX alone (29.2%). Of note, about one-third of the patients who received MTX monotherapy went into remission. The PASI 75 and 90 scores in the combination and MTX monotherapy groups also were impressive (97.1% and 70.6% vs 54.3% and 28.6%, respectively); the safety profile was similar to that reported in previous studies. Because the study was limited by the lack of a placebo arm, evaluating the high response rates in the two treatment groups is difficult.
The effect of MTX on PsA also was examined in the Norwegian NOR-DMARD registry3; 430 MTX-naive patients with PsA were analyzed, and 1218 MTX-naive patients with RA served as the reference population. In the adjusted analysis, the patients with PsA showed less improvement than did those with RA, but most of the disease activity measures and patient-reported outcomes did improve. Two-year retention rates for MTX therapy in patients with RA or PsA were 65% and 66%, respectively.
Reconciling the different results obtained in the RESPOND trial and the NOR-DMARD registry with those observed in the MIPA trial is difficult; the differences most likely are related to study design. A detailed analysis of the data from the MIPA and RESPOND trials will have to await peer-reviewed publication of these two important studies, but their divergent results certainly underscore the need to conduct a randomized placebo-controlled trial to determine the comparative efficacy and safety of MTX and anti–TNF-α agents in PsA similar in design to the Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes study that was performed in RA.4 The time has come to establish the position of MTX in PsA therapy definitively with a well-designed clinical trial that includes the multiple relevant domains so that clinicians can base treatment decisions on high-level evidence and not on data abstracted from RA and psoriasis studies.
Anti–TNF-α agents
In 2009, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) published evidence-based treatment recommendations for PsA.5 The group analyzed the effectiveness and safety of therapies in the 5 key domains of PsA: peripheral arthritis, psoriasis, axial disease, dactylitis, and enthesitis. The anti–TNF-α agents showed the highest effect sizes for management of the various components of PsA, although most trials with DMARDs did not include these key domains as end points.
In the NOR-DMARD registry, patients with PsA who were receiving MTX were compared with patients receiving a TNF-α inhibitor; at 6 months, the latter had a significantly greater reduction in ESR and in DAS28, 36-Item Short Form Health Survey, Modified Health Assessment Questionnaire (HAQ), and patient global assessment scores in spite of having greater baseline disease activity.6 In a subsequent comparison of 1-year retention rates from the same Norwegian registry, survival with TNF-α inhibitor treatment was significantly higher in patients who had PsA or ankylosing spondylitis than in those who had RA.7
Golimumab, a new TNF-α inhibitor, was approved in 2009 for the treatment of patients with PsA. In the pivotal phase 3 trial, this fully human monoclonal antibody against TNF-α was analyzed for efficacy and safety in 405 patients with active moderate to severe PsA.8 Patients were randomized to receive the active drug (50 or 100 mg) or placebo every 4 weeks.
At week 14, 48% of patients who were receiving golimumab achieved an ACR 20 response, compared with 9% of placebo patients. Of patients who entered the trial with 3% or more body surface area, 40% in the 50-mg group and 58% in the 100-mg group achieved the PASI 75 end point. The enthesitis and dactylitis scores at week 24 also were significantly improved. Radiographic progression, measured by the modified van der Heijde-Sharp score, decreased in the group treated with the active drug, a result similar to that seen with other anti–TNF-α medications. The adverse-effect profile was quite good-8.6% of the patients treated with golimumab (34 of 394) experienced serious adverse events through week 104, 8 cases of malignancy (3 were basal cell carcinomas and 1 was fatal lung cancer) were noticed, and 1 case of histoplasmosis was managed successfully.
Abatacept and
rituximab in PsA
Although the use of TNF-α inhibitors has been successful in managing PsA, more than one-third of patients do not achieve the ACR 20 end point in clinical trials and many patients cannot take these medications because of adverse effects or cost constraints. Because abatacept and rituximab have proved effective for patients with RA who have had an inadequate response to anti–TNF-α agents, pilot studies were designed to test their effectiveness in PsA.
Patients with moderate to severe PsA were enrolled in a phase 2, double-blind, randomized, placebo-controlled clinical trial to assess the efficacy of abatacept, a fusion molecule that inhibits T-cell co-stimulation.9 Maintenance therapy with MTX was provided, and the patients were randomized to receive placebo or an escalating dose of the active drug every other week for 3 doses, then monthly. The primary end point was ACR 20 at day 169, with several secondary end points.
The highest ACR 20 response was observed in the 10 mg/kg group (48% vs 19% in the placebo group; P < .006). TNF-α–naive patients responded better to abatacept than patients who previously were exposed to an anti–TNF-α agent, but this difference was not statistically significant. Improvements in HAQ score, physical component summary, and MRI score also were seen with abatacept compared with placebo. The skin response was not impressive; a PASI 50 score was observed in 29% to 43% of patients compared with 14% of those who received placebo. No difference in safety signals was observed among the various treatment arms and the placebo group.
In an open-label pilot study, 20 patients with moderate PsA (14 TNF-α inhibitor–exposed, 6 TNF-α inhibitor–naive) were treated with rituximab, 1000 mg, on days 0 and 15.10 At week 24, the ACR 20, 50, and 70 responses were achieved by 35%, 20%, and 5% of the patients, respectively. Only 30% of the patients achieved the PASI 50 end point. Patients who were not exposed to a TNF-α inhibitor had higher joint responses than patients who had inadequate responses to these agents; these findings are similar to those with abatacept.
The results of these studies are quite intriguing from several perspectives. First, abatacept was not very effective for patients with PsA who had inadequate responses to TNF-α inhibitors-in stark contrast to the findings in RA. Second, the skin response to this agent was significantly less than anticipated on the basis of a previous small pilot study conducted by Abrams and associates11 in which impressive improvements in psoriasis were noted. Third, rituximab does not appear to have a therapeutic role in psoriasis or PsA. These data highlight the different pathophysiological mechanisms that underlie RA and PsA and present a challenge to examine alternative immune and inflammatory pathways for therapeutic targets in psoriasis and PsA.
THERAPEUTIC PIPELINE
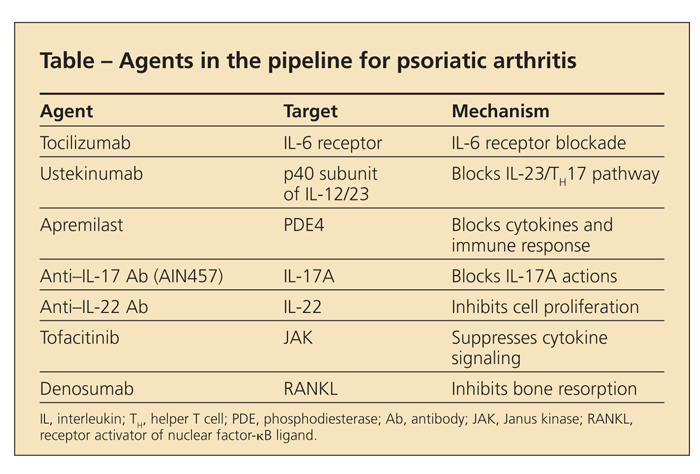
Few options are currently available for patients with PsA who experience an inadequate response or cannot tolerate TNF-α inhibitors, but the pipeline looks very promising (Table). Ustekinumab, an antibody that binds to the p40 subunit of interleukin (IL)-12/23, has been approved for psoriasis and was effective for PsA in a phase 2B trial.12 Tocilizumab, the IL-6R inhibitor, has been approved for RA and is anticipated to be effective for PsA as well.
Apremilast, a phosphodiesterase-4 inhibitor, is in trials in psoriasis and PsA. Tofacitinib, a drug that inhibits Janus kinase, is in phase 2 trials in psoriasis; it is expected to be tested in PsA. Both agents are oral medications.
IL-17 and IL-22 are key cytokines in psoriasis; because recent data indicate that helper T 17 cells produce increased amounts of IL-17 in the blood and joints of patients with PsA, agents against these targets hold great promise in psoriatic disease. In addition, denosumab, an antibody against receptor activator of nuclear factor-κB ligand recently approved for osteoporosis, has the potential to inhibit aggressive bone loss observed in some forms of PsA.
SUMMARY
The number of safe and effective therapeutic options for patients with PsA has increased significantly. In addition, potential new agents hold great promise for the years ahead.
References1. Kingsley GH, Kowalczyk A, Taylor H, et al. Methotrexate is not disease modifying in psoriatic arthritis: the MIPA trial. Arthritis Rheum. 2010;62(suppl 10):S277.
2. Raffayova H, Kungurov N, Kubanova A, et al. Methotrexate nave psoriatic arthritis patients respond rapidly to infliximab plus methotrexate therapy-results from the RESPOND trial. Arthritis Rheum. 2009;51.
3. Lie E, van der Heijde D, Uhlig T, et al. Effectiveness and retention rates of methotrexate in psoriatic arthritis in comparison with methotrexate-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69:671-676.
4. Klareskog L, van der Heijde D, de Jager JP, et al; TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) Study Investigators. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
5. Ritchlin CT, Kavanaugh A, Gladman DD, et al; Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Treatment recommendations for psoriatic arthritis. Ann Rheum Dis. 2009;68:1387-1394.
6. Heiberg MS, Kaufmann C, Rdevand E, et al. The comparative effectiveness of anti-TNF therapy and methotrexate in patients with psoriatic arthritis: 6 month results from a longitudinal, observational, multicentre study. Ann Rheum Dis. 2007;66:1038-1042.
7. Heiberg MS, Koldingsnes W, Mikkelsen K, et al. The comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum. 2008;59:234-240.
8. Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study [published correction appears in Arthritis Rheum. 2010;62:2555]. Arthritis Rheum. 2009;60:976-986.
9. Mease P, Genovese M, Ritchlin C, et al. Abatacept in patients with psoriatic arthritis: results of a phase II study. Ann Rheum Dis. 2010;69:581.
10. Mease P, Genovese M, Ritchlin C, et al. Rituximab in psoriatic arthritis: an open label study. Ann Rheum Dis. 2010;69:116.
11. Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243-1252.
12. Gottlieb A, Menter A, Mendelsohn A, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial [published corrections appear in Lancet. 2010;376:1542; Lancet. 2009;373:1340]. Lancet. 2009;373:633-640.