The primary care physician's guide to inflammatory arthritis: Diagnosis
Inflammatory arthritis includes a variety of diseases; the most common are rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. A large proportion of patients seen in most rheumatology practices have inflammatory arthritis; these patients also are seen in the primary care setting, where they present initially before a diagnosis has been made. The inflammatory arthritides are chronic progressive diseases that can cause irreversible joint damage even early in the disease course.
Significant improvements have been seen in the diagnosis and management of the inflammatory arthritides over the past 10 years, including new diagnostic tests and radiological studies with higher sensitivity and specificity and biologic therapies with various mechanisms of action and improved ease of administration. The most common inflammatory arthritides-rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriatic arthritis (PsA)-are chronic conditions with varying causes and clinical presentations. If not recognized and managed early in the disease course, these disorders may result in irreversible joint destruction, significant physical disability, and comorbidities.1,2
The inflammatory arthritides present a diagnostic challenge to both rheumatologists and primary care physicians. Inflammatory arthritis accounts for a large proportion of the patients seen in most rheumatology practices. Patients with these diseases also may be seen in the primary care setting, often presenting there before a diagnosis has been made. In fact, musculoskeletal complaints-among the most common ones that primary care physicians encounter-may be manifestations of an underlying inflammatory arthritis. Therefore, primary care physicians need to be able to recognize inflammatory arthritis and then consider referral to a rheumatologist for definitive treatment. Comanagement between the primary care physician and the rheumatologist may play a key role in the control of these diseases and their comorbidities.
In this 2-part article, we provide primary care physicians with practical information about the diagnosis and management of the inflammatory arthritides. This first part addresses the clinical presentation and diagnosis. In the second part, to appear in a future issue of this journal, we will describe the latest treatments for patients with these conditions.
CLINICAL PRESENTATION
AND DIAGNOSIS

In 2006, the European League Against Rheumatism published a report about the importance of recognizing and treating early arthritis and proposed 12 useful recommendations for early management (Table 1).3 Among them is referral of patients who present with arthritis of more than 1 joint to a rheumatologist, ideally within 6 weeks of onset of symptoms.
Rheumatoid arthritis
RA is the most common form of chronic systemic inflammatory disease; it affects 0.5% to 1% of adults in the United States.4 Some data suggest that the incidence of RA is increasing.5
RA may occur at any age but usually presents during the fourth and fifth decades of life. Women are affected more often than men and experience a greater degree of disease activity, pain, functional disability, and psychological distress. In some ethnic groups, including American Indians (Pima or Chippewa) and Alaska Natives, the prevalence of RA is higher than it is in white persons.6
Patients with RA often present with an insidious onset of joint pain and swelling. The wrists, metacarpophalangeal (MCP) joints, proximal interphalangeal (PIP) joints of the fingers, interphalangeal joints of the thumbs, and metatarsophalangeal (MTP) joints are affected most often. The joints of the knees, elbows, shoulders, and ankles are less frequently affected; involvement of these joints usually indicates progressive disease activity and severity. The more common extra-articular manifestations of RA include morning stiffness, fatigue, weight loss, inflammatory eye disease (eg, scleritis), vasculitis, and interstitial lung disease. Refer patients with suspected RA to a rheumatologist early, especially if they have 3 or more swollen joints, MCP and MTP joint involvement, or morning stiffness lasting 30 minutes or longer.7
The diagnosis of RA entails a combination of appropriate clinical features and supporting diagnostic studies. Rheumatoid factor (RF) is a more sensitive test, and anti–cyclic citrullinated peptide (anti-CCP) antibody is a more specific test. RF has a sensitivity and specificity of 69% and 85%, respectively; the anti-CCP antibody has a sensitivity and specificity of 67% and 95%, respectively.8,9 A small number of patients with RA will remain seronegative throughout the course of their disease. For the diagnosis of very early RA, positive results on both RF and anti-CCP antibody tests are more useful than a positive result on either test alone.10 RF and anti-CCP are not only diagnostic clues but also prognostic indicators, because RF and especially anti-CCP are associated with more aggressive disease associated with structural damage.
Anti-modified citrullinated vimentin, a newer test developed for the diagnosis of early RA, has a reported sensitivity of 71%, with a specificity of 95%.11 However, this test is not widely available. Inflammatory biomarkers (eg, erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP] level) may provide information about disease activity and response to treatment, but they are not disease-specific.

The 1987 revised American College of Rheumatology criteria for the classification of RA are sensitive and specific in patients who have had symptoms for more than 2 years (Table 2).12 However, these criteria are not as useful if symptoms have been present for less than 1 year. A new classification for RA is under development to include newer diagnostic studies, such as anti-CCP antibody and imaging studies (eg, ultrasonography and MRI) to detect synovitis and erosion. It should be remembered that classification criteria are used to ensure specificity of diagnosis for clinical studies and often do not perform well as diagnostic criteria, especially early in the disease.
Plain radiographs may show evidence of RA early in the disease course and may help the diagnosis. Relevant findings might include erosions at the margins of the joints, especially of the MCP and PIP joints, the wrists (ulnar styloid), and the MTP joints. Because radiographic erosions often occur earlier in the feet, initial imaging should include plain radiographs of the hands, wrists, and feet.13 Also, radiological imaging techniques, including ultrasonography and MRI, are more sensitive for detecting synovitis and erosions in early RA and may be useful when the diagnosis is in question.13,14
The importance of early referral cannot be overstated. In patients who have RA, a delay of 6
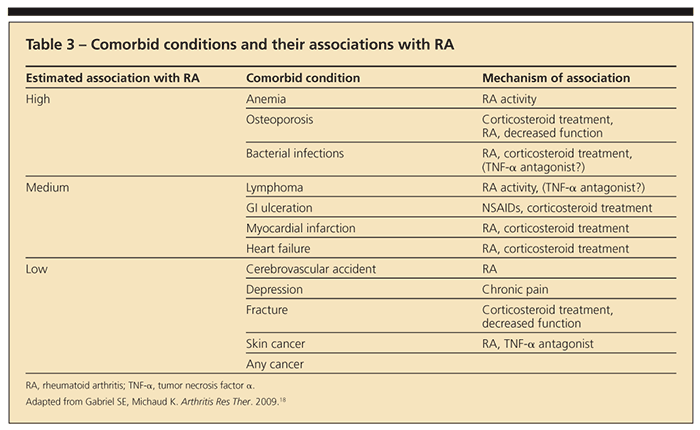
months in the institution of disease-modifying treatment may result in irreversible joint destruction. By the time synovitis is detected clinically, it is usually chronic and joint damage may have already occurred.15-17 The goal of therapy in RA is to treat to remission, which means reducing synovitis and disease activity to the lowest possible level. This requires constant monitoring of patients with RA for evaluation of disease activity and treatment-related toxicity and adjusting therapy as dictated by these parameters. In addition, RA is associated with multiple comorbidities that increase rates of morbidity and mortality in patients with the disease (Table 3).18
Ankylosing spondylitis
In addition to its classification as an inflammatory arthritis, AS is one of the spondyloarthropathies (SpA), which also include PsA, reactive arthritis, and inflammatory bowel disease (IBD)–associated SpA. AS is the prototypical SpA, with a prevalence of 0.5% to 1.0% among white persons in the United States and the United Kingdom, 0.1% in some African populations, and as high as 6% in the Haida Indians of the Pacific Northwest Coast.18-23 The prevalence of AS in a given population correlates with the prevalence of the HLA-B27 gene in that population. The HLA-B27 gene is present in populations throughout Europe and Asia but is virtually absent among the genetically unmixed native populations of South America, Australia, and some regions of equatorial and southern Africa.
AS frequently manifests during the second decade of life. Symptoms rarely appear before the age of 10 to 12 years or after age 40 years. Patients often present with back pain that primarily involves the sacroiliac joint.22 Back pain caused by AS is inflammatory in nature and is characterized by the following parameters (the presence of at least 4 of 5 parameters has a sensitivity of 77% and a specificity of 92%24):
•Onset at 40 years or younger.
•Insidious in onset and present for at least 3 months.
•Morning stiffness is present and improves with exercise.
•Pain occurs at night.
•No improvement with rest.
Back pain is a common complaint among patients in the primary care setting, and up to 5% of these patients may have inflammatory back pain.25 Patients also may complain of pain in the buttocks that often alternates between the right and left sides and is severe enough to disrupt sleep. Extra-spinal features associated with AS include peripheral arthritis; enthesitis, or inflammation at sites of tendinous or ligamentous attachments to bone, such as the Achilles tendon, plantar fascia, and patellar tendon; dactylitis, also called sausage digit; uveitis; and IBD.26 As does RA, AS has associated comorbidities, specifically cardiovascular disease and overall diminished quality of life.27
The diagnosis of AS is made by a combination of clinical history, physical examination, radiological studies, and ancillary blood tests.28-31 Spinal mobility is assessed conventionally using the Schober test and occiput-to-wall distance. Measurement of chest expansion is useful in determining the degree of costovertebral joint involvement.
The Schober test is a method of measuring spinal mobility by marking a length of 10 cm in the lumbar spine starting at the fifth lumbar spinous process and extending upward. The patient then bends forward maximally. The marked distance should increase to at least 15 cm. A distance less than 15 cm indicates reduced spinal mobility.
The occiput-to-wall distance is determined by having the patient stand with his or her back to the wall and then try to touch the back of his head to the wall. Any gap indicates thoracic or cervical disease or both.
Measurement of chest expansion is performed using a tape mea-sure wrapped around the patient's chest at the fourth intercostal space in men or just below the breast in women. Expansion of less than 5 cm is abnormal.
Patients with AS may have anemia (15%); elevated inflammatory markers (ESR and CRP level), often without a definite correlation with disease activity (75%); and elevated alkaline phosphatase levels.32 Screening for HLA-B27 in all patients with back pain is not useful because more than 95% of these patients do not have a spondyloarthropathy. In patients with inflammatory back pain or sacroiliitis on plain radiographs, the addition of a positive HLA-B27 result increases the likelihood of a diagnosis of an axial spondyloarthropathy. The diagnostic specificity of the HLA-B27 test increases with an associated decrease of prevalence in the target population.
The best predictor of whether AS will develop subsequently is radiographic evidence of sacroiliitis coupled with clinical assessment.31 Initial radiological imaging should include plain radiographs of the pelvis and lumbar spine.33 In some instances, oblique views of the sacroiliac joints may improve visualization. Radiographic changes in the sacroiliac joint occur progressively as erosions, sclerosis, narrowing, and fusion.
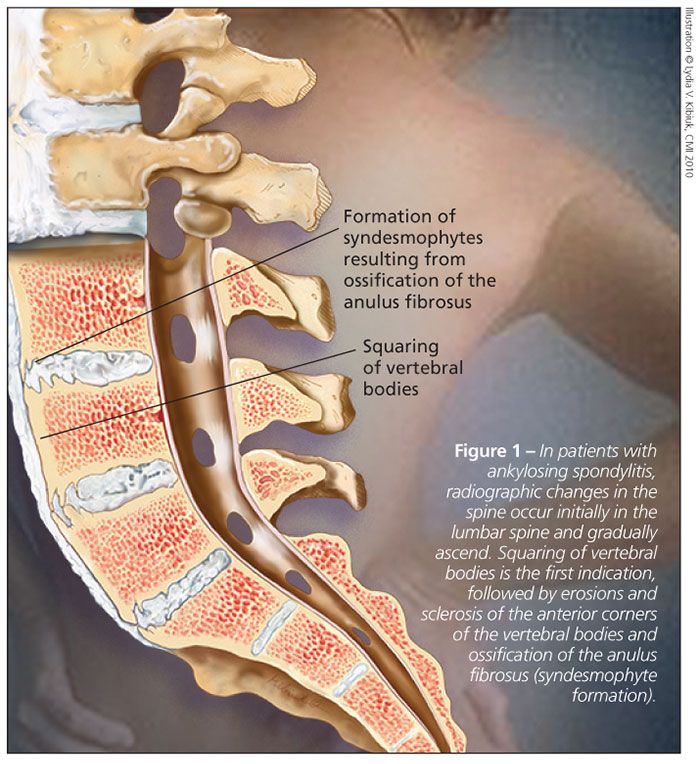
Radiographic changes in the spine occur initially in the lumbar spine and gradually ascend the spine, generally in a continuous fashion. The first indication is squaring of vertebral bodies (Figure 1), followed by progression to erosions and sclerosis of the anterior corners of the vertebral bodies (“shiny corners”), ossification of the anulus fibrosus (syndesmophyte formation) and, over many years, spinal fusion (“bamboo spine”).
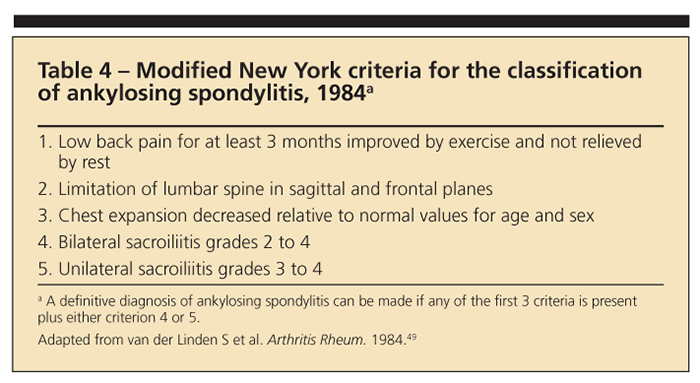
MRI is the most sensitive imaging technique for detection of the inflammatory sacroiliac and spinal lesions of AS.34 MRI with short tau inversion recovery may detect bony edema resulting from inflammation in the sacroiliac joints and spine, one of the earliest abnormalities in AS.35 Joint and bone abnormalities related to AS may be detected much earlier on MRI scans than on plain radiographs, potentially allowing for treatment initiation before significant structural damage has occurred. When the modified New York criteria for classification of AS (Table 4) and plain radiographs are relied on for the diagnosis of AS, it often is delayed for years.36,37 New criteria for the classification of AS probably will include recommendations for the use of MRI.
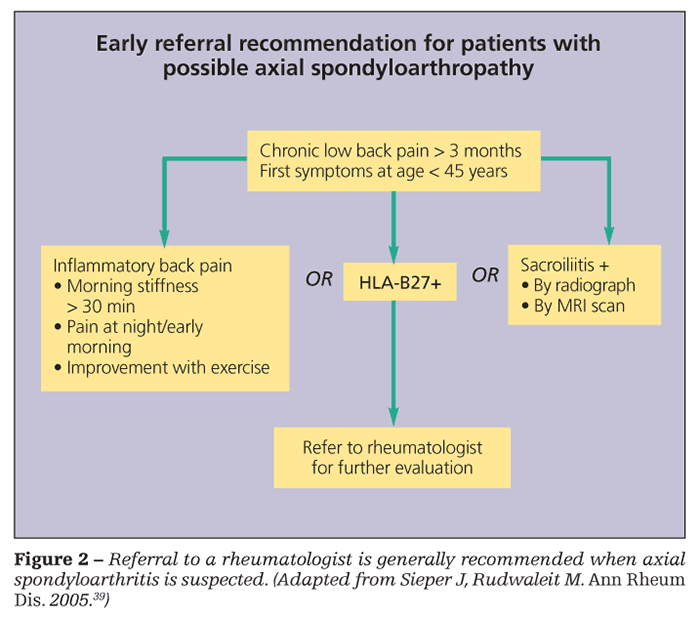
Ultrasonography may be feasible and cost-effective for assessing peripheral inflammation, especially enthesitis.38 Recommendations for evaluation and referral to a rheumatologist for patients with suspected axial SpA are shown in Figure 2.39
Psoriatic arthritis
Erosive and destructive, PsA results in diminished functional capacity and poor quality of life, similar to RA.40 Psoriasis has an overall prevalence of about 2% in the US population.41 Of patients with psoriasis, PsA occurs in about 20% (range, 6% to 42%).42 Generally, the skin disease occurs before or simultaneously with the arthritis. However, in a significant minority of patients, the arthritis precedes the skin disease, resulting in diagnostic difficulty. Both psoriasis and PsA have been associated with the metabolic syndrome; heart disease, including congestive heart failure; hypertension; type 2 diabetes mellitus; dyslipidemia; and peripheral vascular disease.18
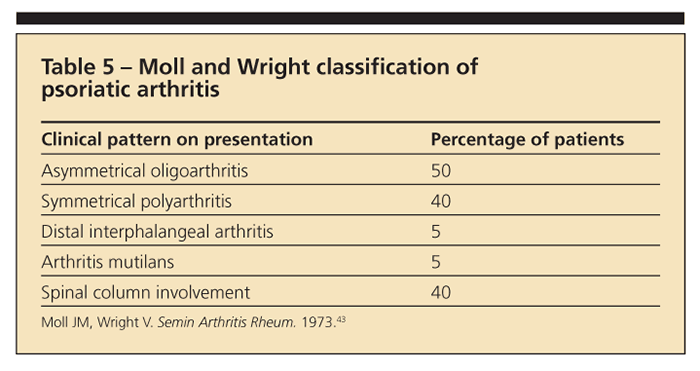
Spondylitis is present in up to 40% to 50% of patients with PsA; therefore, PsA is classified as an SpA.42 Patients with PsA present with typical psoriatic lesions (ie, over the elbows, knees, scalp, and gluteal cleft region). Nail lesions, especially with pitting, often are associated with PsA and are a distinguishing characteristic not seen in RA. The clinical patterns of PsA, as described by Moll and Wright,43 are highlighted in Table 5.
PsA presents more often as an oligoarthritis (involving 4 or fewer joints) than as a polyarthritis (involving 5 or more joints). However, polyarthritis ultimately develops in most patients.42,44 Distal interphalangeal (DIP) arthritis and arthritis mutilans patterns of PsA are uncommon. Pain and synovitis of the wrists and the MCP, PIP, and DIP joints are common.44 Spinal disease may occur with other patterns of PsA or without peripheral arthritis. Symptoms of axial skeletal involvement are nocturnal back pain and morning stiffness.45
Other features of PsA include enthesitis and dactylitis; the latter occurs in about one-third of
patients with PsA. In addition to skin and nail involvement, extra-articular manifestations of PsA include uveitis and conjunctivitis; cardiac abnormalities are rare.44
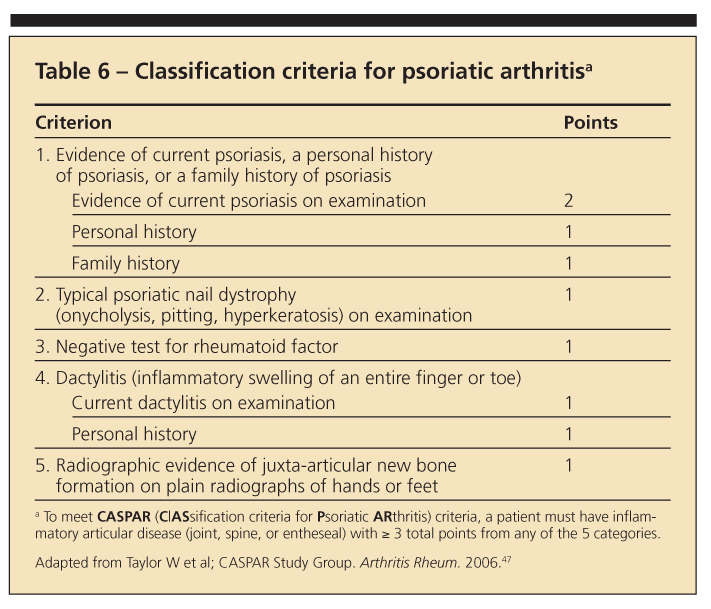
Differentiating PsA from RA may be difficult because several signs and symptoms overlap. A number of classification criteria have been developed for PsA, although none has been accepted universally.46 Using classification criteria developed in 2005 (Table 6),47 a score of 3 or more points is 98.7% sensitive and 91.4% specific for the diagnosis of PsA.
PsA should be considered in any patient who has articular symptoms, especially if characteristic psoriatic lesions are present. However, not all patients with arthritis and psoriasis have PsA. Prominent involvement of the DIP joint is characteristic of PsA and rarely is present in osteoarthritis or RA. Inflammatory spinal involvement also is suggestive of PsA.42,44
MRI scans of the axial skeleton may show inflammatory lesions of the sacroiliac joints and spine at an early stage.35 Musculoskeletal ultrasonography may be useful in the diagnosis of synovitis, enthesitis, and tenosynovitis and in monitoring response to therapy, assessing structural joint damage, and guiding injection therapy.38 Power Doppler ultrasonography and microbubble contrast agents may also improve the usefulness of ultrasound for PsA.48
CONCLUSION
The diagnosis and management of the inflammatory arthritides present a significant challenge. The importance of early recognition of the signs and symptoms cannot be overemphasized.
References:
References1. Bergman MJ. Social and economic impact of inflammatory arthritis. Postgrad Med. 2006 May;Spec No: 5-11.
2. Coordinating Center for Health Promotion. Targeting Arthritis: Reducing Disability for 46 Million Americans 2008. Atlanta: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2008.
3. Combe B, Landewe R, Lukas C, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007;66:34-45.
4. Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4:130-136.
5. Gabriel SE, Crowson CS, Maradit KH, Therneau TM. The rising incidence of rheumatoid arthritis [abstract]. Arthritis Rheum. 2008;58:S453.
6. Hochberg MC, Spector TD. Epidemiology of rheumatoid arthritis: update. Epidemiol Rev. 1990;12:247-252.
7. Emery P, Breedveld FC, Dougados M, et al. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis. 2002;61:290-297.
8. Alessandri C, Priori R, Modesti M, et al. The role of anti-cyclic cytrullinate antibodies testing in rheumatoid arthritis. Clin Rev Allergy Immunol. 2008;34:45-49.
9. Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797-808.
10. Matsui T, Shimada K, Ozawa N, et al. Diagnostic utility of anti-cyclic citrullinated peptide antibodies for very early rheumatoid arthritis. J Rheumatol. 2006;33:2390-2397.
11. Mathsson L, Mullazehi M, Wick MC, et al. Antibodies against citrullinated vimentin in rheumatoid arthritis: higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 2008;58:36-45.
12. Saraux A, Berthelot JM, Chalès G, et al. Ability of the American College of Rheumatology 1987 criteria to predict rheumatoid arthritis in patients with early arthritis and classification of these patients two years later. Arthritis Rheum. 2001;44:2485-2491.
13. Ãstergaard M, Pedersen SJ, Døhn UM. Imaging in rheumatoid arthritis-status and recent advances for magnetic resonance imaging, ultrasonography, computed tomography and conventional radiography. Best Pract Res Clin Rheumatol. 2008;22:1019-1044.
14. Cyteval C. Doppler ultrasonography and dynamic magnetic resonance imaging for assessment of synovitis in the hand and wrist of patients with rheumatoid arthritis. Semin Musculoskelet Radiol. 2009;13:66-73.
15. Combe B. Early rheumatoid arthritis: strategies for prevention and management. Best Pract Res Clin Rheumatol. 2007;21:27-42.
16. Hitchon CA, Peschken CA, Shaikh S, El-Gabalawy HS. Early undifferentiated arthritis. Rheum Dis Clin North Am. 2005;31:605-626.
17. Mitchell KL, Pisetsky DS. Early rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:278-283.
18. Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11:229.
19. Braun J, Bollow M, Remlinger G, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 1998;41:58-67.
20. De Angelis R, Salaffi F, Grassi W. Prevalence of spondyloarthropathies in an Italian population sample: a regional community-based study. Scand J Rheumatol. 2007;36:14-21.
21. Gran JT, Husby G. The epidemiology of ankylosing spondylitis. Semin Arthritis Rheum. 1993;22:319-334.
22. Sieper J, Rudwaleit M, Khan MA, Braun J. Concepts and epidemiology of spondyloarthritis. Best Pract Res Clin Rheumatol. 2006;20:401-417.
23. Trontzas P, Andrianakos A, Miyakis S, et al; ESORDIG study group. Seronegative spondyloarthropathies in Greece: a population-based study of prevalence, clinical pattern, and management. The ESORDIG study. Clin Rheumatol. 2005;24:583-589.
24. Sieper J, van der Heijde D, Landewé R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
25. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
26. Kataria RK, Brent LH. Spondyloarthropathies. Am Fam Physician. 2004;69:2853-2860.
27. Ward MM, Reveille JD, Learch TJ, et al. Impact of ankylosing spondylitis on work and family life: comparisons with the US population. Arthritis Rheum. 2008;59:497-503.
28. Song IH, Sieper J, Rudwaleit M. Diagnosing early ankylosing spondylitis. Curr Rheumatol Rep. 2007;9:367-374.
29. Elyan M, Khan MA. Diagnosing ankylosing spondylitis. J Rheumatol Suppl. 2006;78:12-23.
30. Rudwaleit M, van der Heijde D, Khan MA, et al. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63:535-543.
31. Eastmond CJ, Robertson EM. A prospective study of early diagnostic investigations in the diagnosis of ankylosing spondylitis. Scott Med J. 2003;48:21-23.
32. van der Linden S, van der Heijde D, Maksymowych WP. Ankylosing spondylitis. In: Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley's Textbook of Rheumatology. Philadelphia: Saunders Elsevier; 2009:1169-1189.
33. Maksymowych WP, Landewé R. Imaging in ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2006;20:507-519.
34. Maksymowych WP. MRI in ankylosing spondylitis. Curr Opin Rheumatol. 2009;21:313-317.
35. Maksymowych WP. Imaging in spondyloarthritis. Adv Exp Med Biol. 2009;649:17-36.
36. Dincer U, Cakar E, Kiralp MZ, Dursun H. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008;27:457-462.
37. Feldtkeller E, Khan MA, van der Heijde D, et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61-66.
38. Sturrock RD. Clinical utility of ultrasonography in spondyloarthropathies. Curr Rheumatol Rep. 2009;11:317-320.
39. Sieper J, Rudwaleit M. Early referral recommendations for ankylosing spondylitis (including pre-radiographic and radiographic forms) in primary care. Ann Rheum Dis. 2005;64:659-663.
40. Sokoll KB, Helliwell PS. Comparison of disability and quality of life in rheumatoid and psoriatic arthritis. J Rheumatol. 2001;28:1842-1846.
41. Myers WA, Gottlieb AB, Mease P. Psoriasis and psoriatic arthritis: clinical features and disease mechanisms. Clin Dermatol. 2006;24:438-447.
42. Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(suppl 2):ii14-ii17.
43. Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55-78.
44. Gladman DD. Psoriatic arthritis. Dermatol Ther. 2009;22:40-55.
45. Gladman DD, Strand V, Mease PJ, et al. OMERACT 7 psoriatic arthritis workshop: synopsis. Ann Rheum Dis. 2005;64(suppl 2):ii115-ii116.
46. Helliwell PS, Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis. 2005;64(suppl 2):ii3-ii8.
47. Taylor W, Gladman D, Helliwell P, et al; CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665-2673.
48. Klauser A, Halpern EJ, Frauscher F, et al. Inflammatory low back pain: high negative predictive value of contrast-enhanced color Doppler ultrasound in the detection of inflamed sacroiliac joints. Arthritis Rheum. 2005;53:440-444.
49. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-368.