Guselkumab vs Adalimumab in Plaque Psoriasis
Guselkumab, a selective antagonist of IL-23, went head to head with adalimumab in a year-long phase 2 trial of 293 patients with moderate to severe plaque psoriasis. A quick synopsis of the results here.
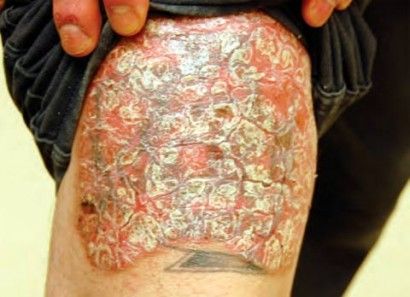
Plaque psoriasis. (Tanya Munger, MSN, Robert Bales, MD, MPH)
Gordon KB, Duffin KC, Bissonnette R, et al. A Phase 2 Trial of Guselkumab versus Adalimumab for Plaque Psoriasis. N Engl J Med. 2015; 373:136-144. July 9, 2015. DOI: 10.1056/NEJMoa1501646
Guselkumab was significantly more effective than adalimumab in patients with moderate-to-severe plaque psoriasis, in a 52-week phase 2 trial. Adverse effects were similar, but the study was not powered to measure serious adverse effects.
The primary end point was the proportion of patients with Physician’s Global Assessment (PGA) score of 0 or 1 at week 16. The trial continued to evaluate efficacy at week 40 and to evaluate adverse effects in a followup to week 52. A total of 293 patients with moderate to severe plaque psoriasis (PGA score mostly 3 and 4, with some 5) were randomized to placebo, to 1 of 5 doses of guselkumab, or to adalimumab. At week 16, patients in the placebo group crossed over to receive guselkumab.
At the primary end point, week 16, more patients in the 4 higher dosages of guselkumab had PGA 0 or 1 than in the adalimumab group.
Patients reaching PGA 0 or 1
GUS = guselkumab, ADA = adalimumab
† = primary end point
* = statistically significant compared to ADA
Guselkinumab is an antibody to interleukin-23 (IL-23). IL-23 is upstream of the IL-17/T helper 17 (Th17) axis, which drives differentiation of Th17 cells. So guselkumab targets a critical pathway. In contrast, ustekinumab targets both IL-23 and IL-12. So guselkumab is a selective antagonist of IL-23.