New Treatment Option for Behçet’s Disease
On Friday, the U.S. Food and Drug Administration approved apremilast (Otezla, Celgene) for the treatment of oral ulcers associated with Behçet’s disease, a rare, chronic, multisystem inflammatory condition that affects approximately five in 100,000 people in the U.S.
Oracl Ulcer (©Leungchopan,AdobeStock)
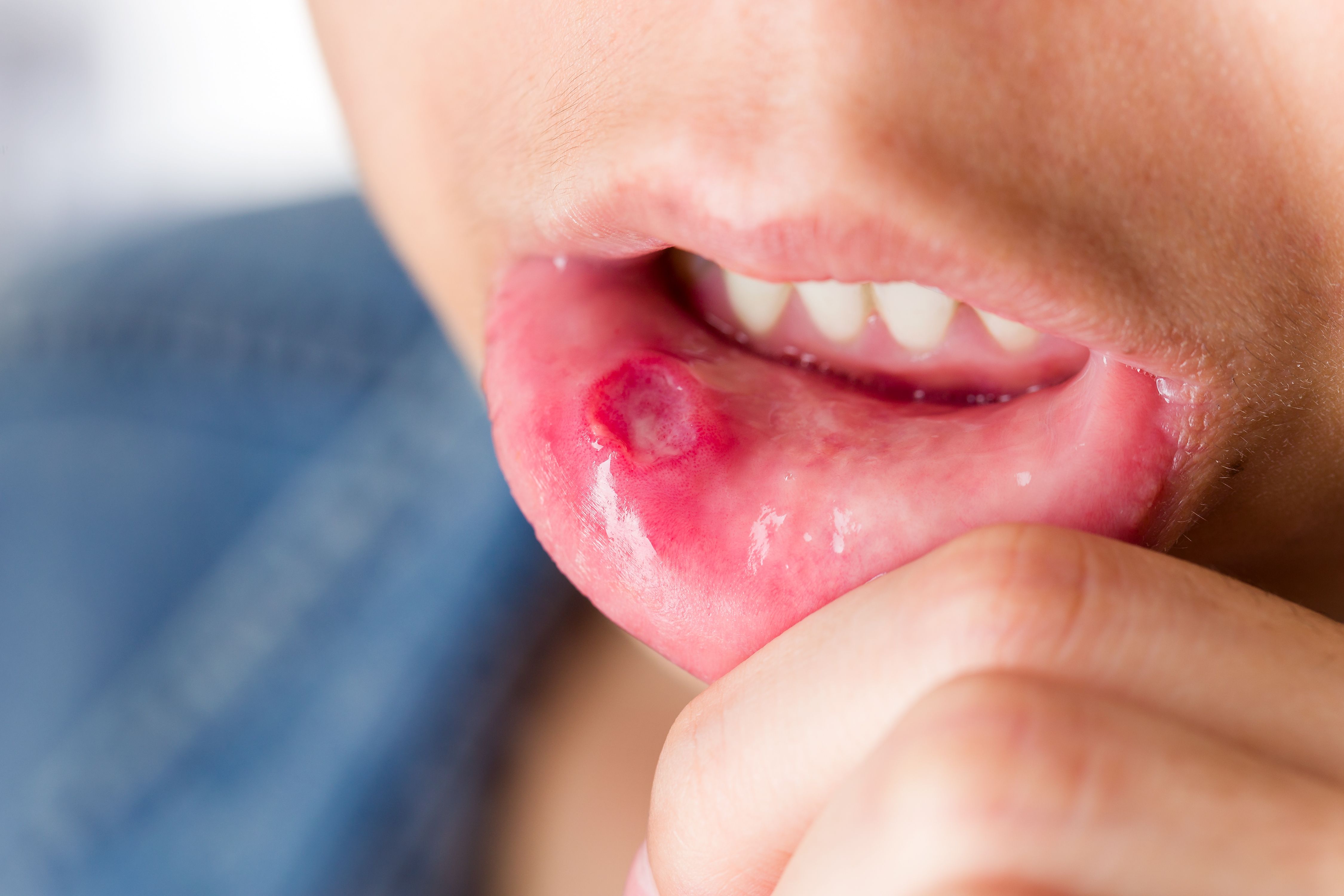
On Friday, the U.S. Food and Drug Administration approved apremilast (Otezla, Celgene) for the treatment of oral ulcers associated with Behçet’s disease, a rare, chronic, multisystem inflammatory condition that affects approximately five in 100,000 people in the U.S.
It is the first and only approved treatment option for oral ulcers associated with Behçet’s disease.
“Oral ulcers are a recurring and debilitating manifestation that affects nearly everyone living with Behçet’s disease, and have an important negative impact on the quality of life for these patients,” said Yusuf Yazici, M.D., of New York University Langone Health. “In the clinical trial, apremilast demonstrated improvements in measures of oral ulcers at week 12. Apremilast has the potential to be a needed treatment option for U.S. patients and their physicians, who previously had limited options available.”
Also known as Behçet’s syndrome, the condition is characterized by recurrent oral and genital ulcers, skin lesions, uveitis arthritis, and vascular, central nervous system and gastrointestinal involvement. Of this, oral ulcers are the most common manifestation of the condition occurring in more than 98 percent of patients.
RELATED: How much do you know about Behcet's disease
Apremilast, which is an oral, selective inhibitor of phosphodiesterase 4 (PDE4), was approved at 30 mg twice daily for adult patients.
The FDA approval is based on the results of a 64-week long randomized, placebo-controlled, double-blind phase 3 trial of 207 adult patients from 53 sites in 10 countries. The patients, who had been previously been treated with at least one nonbiologic and were candidates for systemic therapy, were randomized to a treatment group or placebo (104 and 103 patients respectively).
By week six, 29.8 percent patients achieved a complete response and remained oral ulcer-free for at least six additional weeks during the 12-week treatment phase. By week 12, 52.9 percent of patients who received the treatment (30 mg twice daily) were free of oral ulcers as compared to 22.3 percent in the placebo group. The daily average number of oral ulcers during the 12-week treatment phase was 1.5 in the treatment arm and 2.6 in the placebo arm (based on oral ulcer counts measured at baseline and at weeks 1, 2, 4, 6, 8, 10 and 12).
RELATED: Behcet's Disease Patients Prone to Thrombosis
The most common adverse events occurring in more than 10 percent of patients were diarrhea (41.3 percent), nausea (19.2 percent), headache (14.4 percent) and upper respiratory tract infection (11.5 percent. The safety profile was consistent with the known safety profile of apremilast.
Apremilast is now approved for three indications in the U.S., including the moderate to severe plaque psoriasis for patients who are candidates for phototherapy or systemic therapy; adults with active psoriatic arthritis and now, adults with oral ulcers associated with Behçet’s Disease.
See full prescribing information here.