Management of Lupus in 2010: How Close are the Biologics?
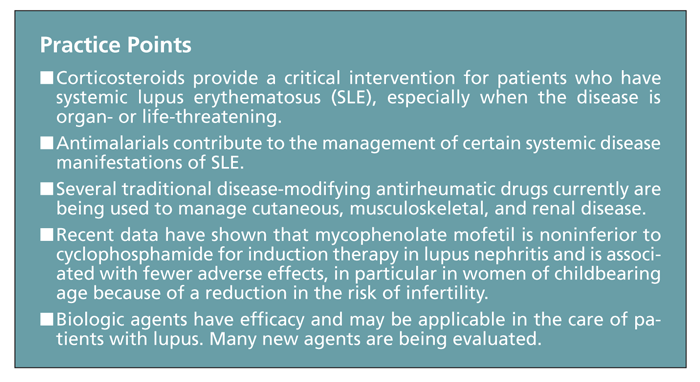
The classic therapies for patients with systemic lupus erythematosus (SLE) often have significant toxicity. Corticosteroids provide a critical intervention when the disease is organ- or life-threatening, but the potential adverse effects are numerous.
Systemic lupus erythematosus (SLE) is a complex autoimmune disease that can affect nearly any organ system and has a variety of clinical presentations. Patients may have symptoms or complications of the skin, joints, serosal surfaces, kidneys, CNS, hematological components, heart, and mucous membranes. SLE typically affects women of childbearing age, but it may be diagnosed in children, men, and women older than 60 years. A characteristic feature is the presence of antibodies against self-antigens, or autoantibodies, which are produced by autoreactive B cells that have received T-cell help. These antibodies deposit in tissues of various organs and induce local inflammation, altered organ function, or both.1
Although treatment has improved survival significantly during the past 40 to 50 years, many patients with lupus still have disease exacerbations that are unresponsive to conventional treatment. They also continue to experience complications, such as infections related to their immunosuppressive therapy, as well as accelerated atherosclerosis and cognitive impairment that appear with disease progression.1
Improved understanding of the pathogenesis of SLE and new insights into the mechanisms of dysregulation of the immune system have led to the development of novel drugs that aim to target, in a more specific manner, the various disease pathways that are involved. Application of these more specific treatments differs from the traditional therapeutic approach to autoimmune disease, which includes a varying combination of anti-inflammatory and cytotoxic drugs. The more targeted approach with biologic agents can potentially treat the disease at the cytokine and cellular level, as is currently being done for several other autoimmune diseases.
In this article, we provide a 2010 update of our previous review published in this journal,2 summarizing important aspects of drug treatment for patients with lupus. We provide a discussion of some of the current therapies and move on to novel approaches in development while touching on selected management strategies. More details about current SLE therapy, including usual doses and adverse effects, can be found elsewhere.3,4
MONITORING AND
TREATMENT
Patients with SLE may present in different ways and with differing levels of severity. The pattern of disease and patient prognosis may be difficult to determine early on, making it crucial to have frequent follow-up and monitoring of symptoms, laboratory data, and disease activity indices.
The frequency of visits depends on the activity, severity, and extent of SLE; response to treatment; type of treatment; and need for toxicity monitoring. At our center, we monitor patients with lupus at 4- to 8-week intervals and at increased frequency during periods of disease activity. At routine visits, complete blood cell (CBC) count, blood chemistry, urinalysis, urinary protein and creatinine levels, anti–double-stranded DNA (dsDNA) antibodies, and complement levels should be assessed, even in patients with previously normal values. If patients are being treated for pericardial or pleural effusions, appropriate imaging should be performed as well. The purpose of this close monitoring is to prevent or diagnose organ damage before it is irreversible.
The recognition of a higher incidence of hypertension, dyslipidemia, diabetes mellitus (DM), atherosclerosis, osteonecrosis, and certain malignancies in patients with SLE in observational studies led the European League Against Rheumatism (EULAR) Standing Committee for International Clinical Studies to recommend diligent follow-up and screening for these comorbidities. It is well understood that patients with lupus have an increased risk of death compared with age-matched controls.3 A recent study that assessed risk factors for coronary artery disease in patients with SLE highlighted the need for clinical vigilance to identify modifiable risk factors in the clinical setting and, in particular, in men.5
For these reasons, careful attention to screening tests, risk factor modification, and management of coexisting diseases is extremely important. In addition, screening with dual-energy x-ray absorptiometry and prevention and management of osteopenia/osteoporosis are essential aspects of lupus management, especially for patients who are currently being treated or who previously had been treated with corticosteroids.3
DISEASE ACTIVITY SCALES, DAMAGE INDICES
In addition to assessing new or changing clinical or serological manifestations at follow-up visits, physicians may use disease activity scales to predict or prevent future flares. For example, the Systemic Lupus Disease Activity Index (SLEDAI), Systemic Lupus Activity Measure, European Consensus Lupus Activity Measure (ECLAM), and British Isles Lupus Assessment Group (BILAG) were developed for monitoring patients in routine clinical practice and clinical trials. These disease activity scores are thought to be helpful in rigorously quantifying disease activity, monitoring response to therapy and, perhaps, predicting flares. The 2008 recommendations of the EULAR Task Force on SLE management advised routine use of at least 1 of these indices in practice.3
In 1992, the Systemic Lupus International Collaborative Clinics (SLICC) and American College of Rheumatology (ACR) developed an organ damage index for SLE, the SLICC/ACR Damage Index.6 The SLICC/ACR Damage Index score attempts to quantify the cumulative disease burden, measuring irreversible damage resulting from SLE disease activity and its treatment. Early renal damage quantified by this score has been shown to predict high rates of morbidity and mortality.7 It is considered a valid means of targeting patients at high risk, and it may be used as a guide for escalation of therapy.
CURRENT THERAPIES
General principles
All patients with SLE should be advised to avoid possible disease triggers, such as sun exposure, high-dose estrogen therapies, and sulfa drugs (up to 30% of persons with lupus are sulfa-allergic8). Also, patients should be counseled on prevention of atherosclerosis and osteoporosis; patients with antiphospholipid antibody syndrome should avoid unnecessary surgeries and vascular catheterizations as well as exogenous estrogen; if they are at high risk, they might benefit from aspirin therapy. Infections must be managed promptly, and if they are severe, immune suppression should be held. Hypothyroidism, metabolic disturbances, myopathy, anemia, and depression may be factors contributing to fatigue that are amenable to treatment.
Corticosteroids
These agents provide a critical intervention for patients with lupus, particularly when the disease is organ- or life-threatening. Therapeutic doses vary with the organ involved. Heart, brain, kidney, or hematological involvement usually requires a high dose of corticosteroids (1 mg/kg of prednisone or equivalent, and sometimes more); in contrast, even severe flares in other systems, such as the skin, joints, or serosal tissues, often can be controlled with a lower dose (0.5 mg/kg or less of prednisone or equivalent).
However, the adverse effects of corticosteroid treatment, including weight gain, emotional lability, myopathy, osteonecrosis, osteoporosis, hypertension, hyperlipidemia, DM, glaucoma, and increased risk of infection, are numerous and relatively common. In addition, patients with SLE who receive long-term prednisone therapy are at significant risk for morbidity resulting from permanent organ damage.9 Whether SLE activity or the corticosteroid treatment is the major contributor to specific types of organ damage is unclear, but long-term corticosteroid therapy can be hazardous and should be undertaken with caution. Therefore, corticosteroids often are used to achieve immediate relief and stabilization of organ function and are then tapered after institution of slower-acting agents (eg, antimalarials or immunomodulatory therapies).10
When long-term (ie, longer than 6 months11) corticosteroid therapy is necessary, osteoporosis is a major cause for concern. Therefore, the ACR has suggested prescribing bone-protecting agents, such as calcium and vitamin D, for all patients being treated with corticosteroids at a dosage of 5 mg/d or more of prednisone or equivalent. In addition, patients treated with corticosteroids for 3 months or longer also should receive bisphosphonates; however, medications from this class are not typically recommended in women of childbearing age and are not safe for use in pregnancy. Annual bone density measurement for all patients undergoing corticosteroid treatment is recommended.11 At conventional doses, topical corticosteroid creams are useful in managing cutaneous lupus manifestations, and they are associated with fewer systemic adverse effects.
Disease-modifying agents
Antimalarials. The benefit of antimalarial agents (eg, hydroxychloroquine [HCQ], chloroquine, and quinacrine) in management of the articular and cutaneous manifestations of SLE is well established.12,13 Evidence has shown that antimalarials also may contribute to management of the systemic disease manifestations by maintaining remission, preventing major disease flares, and reducing the required prednisone dose for patients with SLE.
The Lupus in Minorities: Nature Versus Nurture study has further shown that treatment with HCQ independently reduces the risk of damage accrual in patients without damage at the time of treatment initiation13-16 and may specifically protect against renal damage.17 In addition, data from the multiethnic, international Grupo Latino Americano de Estudio del Lupus Eritematoso cohort demonstrated that patients with lupus using antimalarials have reduced mortality compared with nonusers, possibly in a time-dependent manner.18
Antimalarials increase pH within intracellular vacuoles, thereby altering a number of cellular functions. Among these processes is the formation of peptide–major histocompatibility complexes required to activate T cells, thus potentially modulating the immune response against autoantigenic peptides.19
Another possible mechanism of action of antimalarials in lupus involves modulating the function of plasmacytoid dentritic cells (pDCs). pDCs, via receptors for the Fc portion of IgG (Fcγ Rs) present on their surface, bind immune complexes and transport both the autoantibody and the autoantigen to the cytoplasmic compartment that contains Toll-like receptor 9 (TLR9). Signaling via TLR9, which recognizes DNA-containing immune complexes, leads to the production of interferon and maturation of dendritic cells-a pivotal antigen-presenting cell in SLE pathogenesis.
Chloroquine compounds have been determined to interfere with TLR9 activation in pDCs. HCQ, by decreasing TLR signaling, reduces the activation of dendritic cells, thus mitigating the inflammatory process.20 TLR receptor antagonists, including both chloroquine derivatives and short oligonucleotides sequences, are in development and have demonstrated promise in animal models of SLE.20-22
Most often, antimalarials are used in combination with other treatments; in some mild cases, they may be used alone. Combinations of antimalarial agents appear to be synergistic,12 but this sort of combination is not generally used.
Antimalarials may deposit in the retina, possibly leading to blindness; thus, every patient who receives these medications should undergo annual to biannual eye examinations. However, blindness resulting from antimalarials is somewhat rare, and most vision deterioration is reversible with drug discontinuation.
Of note, antimalarials have been found to be beneficial in improving the lipid profile in lupus. Studies have shown that triglyceride levels and very-low- and low-density lipoprotein cholesterol levels were significantly lower in patients taking antimalarials23; high-density lipoprotein cholesterol levels were elevated.24 A combination of an antimalarial agent with corticosteroids seems to reverse the increased hepatic synthesis of lipoproteins induced by the corticosteroids24 and may have a protective effect against corticosteroid-induced osteoporosis.25
In addition, antimalarials may have mild antiplatelet and antithrombotic effects. Data from the Hopkins Lupus Cohort demonstrate that HCQ reduces the incidence of thrombosis in patients with lupus who have antiphospholipid syndrome.26 Antimalarials also were found to be thromboprotective in another large cohort of patients from the University of Toronto.27 Also, HCQ may reduce antiphospholipid (APL) antibody titers in APL-positive patients with SLE.28
Methotrexate (MTX). Originally used in cancer treatment, MTX is a disease-modifying antirheumatic drug frequently used to control symptoms of rheumatoid arthritis (RA), psoriatic arthritis, and skin psoriasis. Evidence suggests that MTX may be effective in patients with SLE who have articular or cutaneous involvement and limited response to antimalarials and low-dose prednisone and as a corticosteroid-sparing agent in patients in whom it is difficult to reduce the prednisone dose because of articular or cutaneous activity.29,30
MTX is a structural analogue of folic acid. It binds and inactivates dihydrofolate reductase, thus blocking synthesis of purine metabolites. However, this may not be the primary mechanism of action at the low doses used for inflammatory arthritis. MTX inhibits the enzyme aminoimidazole carboxamide ribonucleotide (AICAR) transformylase, leading to increased cellular AICAR concentrations and increased adenosine release, a potent anti-inflammatory/inhibitor of neutrophil function.31
Adverse effects of MTX include cytopenias, hepatitis, alopecia, and stomatitis. In addition, pulmonary complications such as nonproductive cough, dyspnea, and fever with pneumonitis have been noted, making treatment with MTX dangerous in those patients with lupus who have lung involvement.
Azathioprine (AZA). This is a purine analogue that inhibits nucleic acid synthesis and affects both cellular and humoral immune functions. AZA is associated with fewer adverse effects than cyclophosphamide (CYC) and, traditionally, has been considered an alternative in the management of lupus nephritis, particularly for less severe cases.10 AZA also is used frequently as a corticosteroid-sparing agent for nonrenal manifestations,10 and it has proved to be effective in maintaining disease remission.32
The most common adverse effects of AZA are GI intolerance and bone marrow toxicity that requires regular monitoring of CBC counts during therapy. AZA also has been associated with a hypersensitivity syndrome (fever, rash, and elevated hepatic transaminase levels). These adverse reactions typically are reversible with drug discontinuation.10 One study has shown that contrary to common belief, cumulative doses of AZA do not increase the risk of malignancies in patients with SLE.33
Cyclophosphamide. This has long been the gold standard treatment for severe organ-threatening SLE, especially that with renal and CNS involvement,34 although no double-blind studies have proved its efficacy in CNS manifestations. The widespread use of CYC is thought to be a major contributor to the reduction in mortality and end-stage renal failure resulting from lupus nephritis that has occurred over the years.35 At doses required for lupus nephritis, however, CYC is associated with a high rate of adverse events, including gonadal toxicity, malignancies, and infections. Treatment with gonadotropin-releasing hormone may protect against premature ovarian failure when given during CYC therapy.36
The standard CYC treatment regimen for lupus includes monthly intravenous infusions of CYC, 0.5 to 1.0 g/m2 of body surface area, for 6 months to induce remission. Remission is maintained by the continuation of bolus CYC infusions every 3 months for 1 year after remission.37 This regimen was generated from the original NIH trials that demonstrated significant benefit from the addition of CYC to corticosteroids compared with corticosteroids alone for treatment of patients with lupus nephritis. However, lower-dose induction therapy has been studied to reduce the risk of pulsed high-dose CYC treatment.
The Euro-Lupus Nephritis Trial treatment protocol randomized patients to receive 3 intravenous infusions of methylprednisolone at the time of renal biopsy and 6 intravenous infusions of 500 mg of CYC every 2 weeks, followed by AZA as a long-term maintenance therapy, or the standard NIH regimen. That regimen consisted of 6 monthly infusions and 2 additional quarterly infusions of high-dose intravenous CYC adjusted to the white blood cell count nadir, which were then followed by AZA.38
Although the trial was not powered to demonstrate equivalence or superiority, the data have demonstrated that after 10 years, the outcomes in terms of patient and renal survival are similar.39 In addition, maintenance therapy that involves combinations of corticosteroids and AZA or mycophenolate mofetil (MMF)-rather than the quarterly infusions of CYC-has been demonstrated to be safer and, perhaps, more effective.32
Many physicians consider the standard NIH CYC protocol to be effective and the most reliable regimen, in particular for severely ill patients with lupus or multisystem disease. Still, the search continues to find less toxic remission-inducing therapies.
Mycophenolate mofetil. This established, effective immunosuppressive agent is used to prevent and manage renal transplant rejection. Reports also have indicated that MMF is effective in managing lupus nephritis. Less toxic than CYC, MMF has been shown to be equally effective for induction treatment of severe lupus nephritis,40 and it may play a role in managing the nonrenal disease manifestations.
MMF inhibits inosine monophosphate dehydrogenase, the rate-limiting enzyme in de novo purine (guanosine) synthesis. In vitro, MMF blocks B- and T-cell proliferation because these cells depend solely on de novo guanosine synthesis. In addition, depletion of guanosine nucleotides decreases the expression of adhesion molecules and reduces lymphocyte influx to inflammatory sites, such as the kidney.41
Because controlled studies proving its efficacy were lacking, MMF once was used with prudence and typically not as a first-line treatment. This began to change over the past decade. In 2000, Chan and colleagues42 published results from a prospective controlled study that compared the efficacy of MMF and prednisolone with that of oral CYC and prednisolone in inducing remission of diffuse proliferative lupus nephritis. The study demonstrated that MMF was as effective as CYC in inducing remission but with lower risk of the severe adverse reactions associated with CYC.
Contreras and associates32 later demonstrated that MMF or AZA is a more effective and safer maintenance therapy than intravenous CYC. Their study was thought to be more applicable to practice in the United States because its design included an intravenous CYC regimen and its patient population was much more diverse, including a high proportion of African Americans and Hispanics, who are more susceptible to severe renal disease. However, the study measured the efficacy of MMF only as a maintenance drug and not as a remission-inducing agent.
In the Aspreva Lupus Management Study (ALMS), Ginzler and coworkers43 achieved a promising breakthrough in a large multicenter study that demonstrated that induction therapy with MMF is actually superior to intravenous CYC in inducing complete remission of lupus nephritis. Indeed, a recently published meta-analysis of 10 randomized controlled trials involving more than 800 patients with proliferative lupus glomerulonephritis indicated that MMF has efficacy similar to that of CYC in terms of inducing disease remission. However, MMF was not superior to CYC in conferring survival benefit in these patients, although MMF is a safer agent because of its more favorable safety profile.44
In contrast to CYC, MMF generally is well tolerated; most of its adverse effects respond to dose reduction. However, some adverse effects remain common, such as GI intolerance, especially nausea and mild to moderate diarrhea; hematological toxicity35; and an increased risk of infections (although the infections are fewer or at least less serious than those with CYC32,41).
Data on nonrenal disease activity in the study by Ginzler and associates43 comparing MMF and CYC as induction therapy showed that remission could be induced in other organ systems, including cutaneous, musculoskeletal, and cardiovascular systems. This underscores the utility and effectiveness of MMF in lupus.45
High-dose CYC and stem cell transplant. Immunoablation by immunosuppressive therapy has been investigated in the past few years for the management of severe autoimmune diseases. A high-dose CYC regimen may induce maximal immunosuppression by destroying lymphocytes while preserving the bone marrow stem cell population (without myeloablation). Therefore, those latter cells are able to reinitiate a naive immune response. Patients with moderate to severe lupus refractory to corticosteroids and 1 or more immunosuppressive drugs were treated with high-dose CYC without stem cell transplant; a durable complete remission occurred in about 40% of the patients.46
An open-label study showed that treatment with intense immune suppression with the addition of autologous hematopoietic stem cell transplant results in a significant reduction in disease activity.47 Autologous hematopoietic stem cell transplants induced durable, treatment-free remissions in 4 of 8 patients with severe, refractory lupus in an NIH pilot study presented at the 2010 International Congress on Systemic Lupus Erythematosus.48 The 8 patients in this study had not responded to a median of 11 CYC cycles before transplant. Two patients had transverse myelitis, 1 had retinal vasculitis, and 5 had class IV nephritis. Two patients died of treatment-related causes; both had multiple borderline organ dysfunction at the time of enrollment.
The NIH subjects were given granulocyte colony-stimulating factor, along with CYC and rituximab. After retrieval of CD34+ stem cells, patients’ immune systems were ablated with the same dose of rituximab plus CYC and fludarabine. This aggressive protocol was designed to achieve complete remission.
In 2007, Sun and coworkers49 found a therapeutic effect of mesenchymal stem cells (MSCs) in 2 patients with treatment-refractory SLE; they later published the results for these and 14 additional patients who had a lack of response to treatment to monthly intravenous pulse CYC or oral MMF (2000 mg/d for 3 months) and continued daily doses of 20 mg of prednisone or its equivalent. All patients received umbilical MSCs, with or without pretreatment with CYC (patients with severe cytopenias were not given the CYC). Decreased renal disease activity was observed, along with increases in serum albumin, C3, and hemoglobin levels, as well as platelet counts. Although there has yet to be a randomized clinical trial of stem cell transplant in patients with lupus, these preliminary study results are encouraging.48
BIOLOGIC AGENTS
IN SLE THERAPY
Unlike the traditional immunosuppressive therapies, the biologic agents target specific steps in the pathogenesis of SLE (eg, B cells, costimulation, and the cytokine network). Following is a brief overview of biologic agents that show promise in SLE treatment; some are already in advanced clinical trials.
B-cell depletion:
Targeting CD20
Rituximab is a monoclonal antibody (mouse/human chimera) that targets CD20, a cell-surface antigen that is present on B cells in various stages of cell maturation but is absent on plasma cells (antibody-secreting cells). Although rituximab initially was FDA-approved for non-Hodgkin lymphoma, its use for the treatment of patients with SLE has become increasingly prevalent. Rituximab appears to be most useful in patients with severe disease for whom immunosuppressive therapy has not succeeded and in those who have toxic adverse effects or contraindications to standard treatment. It has been shown to be effective for lupus nephritis and neurological impairment, and it may improve other manifestations of lupus (eg, arthritis, serositis, rash, and vasculitis).50
However, recent results in controlled clinical trials of rituximab in lupus have been disappointing. The 52-week Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial tested the efficacy and safety of rituximab versus placebo in patients with moderately to severely active extrarenal SLE. The study showed no difference between rituximab and placebo on any primary or secondary end point, including the patients experiencing a major or partial clinical response, total BILAG score, and time to flare.
A second large trial, LUNAR (the efficacy and safety of rituximab in class III/IV lupus nephritis), compared rituximab therapy with placebo, when added to a background of corticosteroids and MMF. A total of 144 patients were randomized to receive placebo or rituximab in a protocol similar to that in EXPLORER. The results did not show any significant difference in the treatment arms in any primary or secondary outcomes.51 Multiple reasons, including trial design, limitations of outcome instruments, and short follow-up, have been suggested to explain these results.
In contrast to these disappointing results, rituximab reportedly shows effectiveness in clinical experience. A systematic review of off-label rituximab use in refractory multiorgan SLE documented a significant improvement in at least 1 clinical manifestation in most of the patients.52 More recently, the French Autoimmunity and Rituximab registry reported prospective data on 136 patients with SLE treated with rituximab outside of clinical trials; the overall response rate was 71%.53
Conti and colleagues54 used rituximab to treat 23 patients who did not respond to conventional immunosuppressive treatment. More than half received more than 1 cycle of therapy. Six months after the first cycle, patients showed a significant improvement in the mean SLEDAI-2000 and ECLAM scores.
Rare cases of progressive multifocal leukoencephalopathy have been reported in patients receiving rituximab, particularly those with SLE. However, these patients previously had been treated with other immunosuppressive therapies, including CYC, AZA, corticosteroids, intravenous methylprednisolone, and prednisone.55
One protocol suggested for rituximab is a dose of 375 mg/m2 in weekly infusions for 4 weeks in combination with corticosteroids and other immunosuppressive agents.56-58 An alternative regimen of two 1000-mg doses on days 1 and 15 and then 2 additional doses after 6 months has been used. This latter regimen was used in the EXPLORER trial. Usually, patients respond during the first 3 months of treatment in correlation with complete B-cell depletion. Effectiveness may continue even after the recovery of the B-cell count; in cases of relapse after prolonged remission, treatment may be repeated.
Targeting CD22
CD22 is a 135-kd cell surface marker specific to B cells that functions as a coreceptor for the B cell receptor. CD22 modulates B-cell interactions with T cells and plays a key role in B-cell development and survival. It is present on pre–B cells to mature activated B cells but absent on memory B cells.
Epratuzumab is a humanized anti-CD22 monoclonal IgG1 antibody that causes internalization of CD22/antibody complex on binding. Unlike anti-CD20 monoclonal antibodies, epratuzumab does not induce complement-dependent toxicity or apoptosis and only partially (30% to 45%) depletes circulating B cells. Epratuzumab appears to have more of an immunomodulatory than a cytotoxic effect.
In a small, open-label study of 14 patients with SLE, epratuzumab successfully decreased the level of SLE disease activity as measured by BILAG score.59 It was well tolerated, with only mild to moderate infusion reactions. Epratuzumab also has been studied and shown to be effective in primary Sjgren syndrome; current larger trials of epratuzumab in the management of SLE are ongoing.
Costimulatory
interactions
The interaction between B7 on antigen-presenting cells and CD28 on T cells results in T-cell activation, a crucial step in the development of an adaptive immune response. After their activation, T cells start to express cytotoxic T-lymphocyte antigen 4 (CTLA4), which binds to B7 with high affinity, competing with CD28. CTLA4 transduces a negative signal to the T cells, thus down-regulating T-cell activation.
Abatacept (CTLA4-Ig), an antagonist of the activating costimulatory pathway, currently is approved for the treatment of patients with RA who do not respond to tumor necrosis factor (TNF) blockade. Animal data suggest a beneficial role of T-cell costimulation blockade with CTLA4-Ig in murine SLE models.60
A phase 2 exploratory trial evaluated use of abatacept in human nonrenal SLE (discoid lupus, pleurisy, or arthritis). There was some benefit from active treatment, with a 40.7% flare rate in the abatacept group versus 54.4% in the placebo group. However, these results have to be interpreted with caution in view of the post hoc nature of the analysis. Rates of serious adverse events were higher in the abatacept group (19.8%) than in the placebo group (6.8%). Two large, randomized, multicenter, placebo-controlled trials to evaluate the use of abatacept in lupus nephritis in combination with MMF or CYC are ongoing.51
Cytokine blockade
Interleukin (IL)-6. Patients with lupus have elevated serum levels of IL-6, which in some studies correlated with disease activity or with anti-dsDNA levels. Elimination of IL-6 led to a significant decrease in the spontaneous production of immunoglobulin and, specifically, anti-dsDNA antibodies in vitro.60
Tocilizumab, a humanized monoclonal antibody against the α-chain of the IL-6 receptor, prevents the binding of IL-6 to membrane-bound and soluble IL-6 receptor; it currently is approved for management of RA and juvenile arthritis.
A recent phase 1 trial reported on patients who had mild to moderate SLE with unresponsive nephritis or extrarenal manifestations who received 1 of 3 doses of tocilizumab administered intravenously every 2 weeks for 12 weeks, for a total of 7 infusions.61 Response rates and the incidence of neutropenia were high, with reductions in inflammatory markers and autoantibody levels. Disease activity decreased significantly. The trial results provide support for further study of this drug in SLE.
Type 1 interferons (IFNs). These have been implicated in the pathogenesis of SLE. As discussed above, after internalization through Fc receptors, autoantibody-containing immune complexes bind endosomal Toll-like receptors 7 and 9 and stimulate production of type 1 IFN. Type 1 IFN stimulates dendritic cell maturation, which promotes loss of tolerance and generation of autoreactive T cells and B cells, autoantibody production, immune complex formation, and further production of type 1 IFN, thus creating a self-perpetuating cycle of autoimmunity.62
Higher IFN-α levels and type 1 IFN activity are associated with greater disease activity in SLE. A phase 1 dose-escalation trial evaluated the effects of a single dose of the anti–IFN-α monoclonal antibody sifalimumab in SLE. The treatment eliminated overexpression of IFN-inducible genes in a dose-dependent manner and demonstrated beneficial effects on downstream signaling pathways.51
B-lymphocyte
stimulator (BlyS)
This cytokine (also called BAFF, TALL-1, and zTNF4), a member of the TNF-α superfamily, plays an important role in B-cell proliferation and subsequent antibody production. BLyS and the related cytokine A PRoliferation-Inducing Ligand (APRIL) are important survival and growth factors for B cells. BAFF binds to 3 different receptors on B cells: transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI), BAFF receptor (BAFF-R), and B-cell maturation protein (BCMA). APRIL binds only to TACI and BCMA.
BLyS/BAFF are overexpressed in SLE, RA, and Sjgren syndrome; blocking this cytokine was shown to be beneficial in lupus animal models.63 A phase 1 clinical trial of a BlyS antagonist has shown this compound to be biologically active and safe.63 However, belimumab, an antibody designed to inhibit the biologic activity of BlyS, did not achieve its overall primary efficacy end point of reducing signs and symptoms in patients with lupus, as measured by a lupus disease-activity index. Still, although primary end points were not met in the phase 2 trials, belimumab stabilized SLE disease activity over the 52-week trial period. In a subgroup analysis, patients who tested positive for antinuclear antibodies responded better and had fewer disease flares than seronegative patients with SLE.64 More encouraging results were noted in phase 3 trials, in which belimumab-treated patients had less SLE disease activity and fewer flares than placebo-treated patients at both 52 weeks and 76 weeks.64,65 No significant increases in adverse events were noted.66
Several other molecules that interfere with this pathway are being studied. Because BAFF and APRIL both bind to TACI, inhibition of both BAFF and APRIL can be accomplished with a TACI receptor fusion protein (although whether adding APRIL blockade is beneficial is not currently known).
Atacicept is a fusion protein composed of the extracellular domain of TACI fused with the Fc fragment of human IgG1. Several early studies in patients with SLE and RA have shown that atacicept can reduce peripheral blood B cells and serum immunoglobulin without associated toxicity.59 Of some concern, a phase 2 trial using this drug in combination with MMF for lupus nephritis was discontinued because of increased infection rates. However, a phase 2-3 trial of this drug for nonrenal lupus is ongoing.
CONCLUSIONS
SLE encompasses a constellation of signs and symptoms that are classified as a single disease entity. For this reason, there may be no single therapy that would be effective for all manifestations i
n the majority of patients who have the disease. Patients with SLE require frequent follow-up and monitoring of symptoms, laboratory data, and disease activity indices; modification of risk factors for disease comorbidities should not be overlooked.
Common treatments for patients with mild disease manifestations (eg, those in the skin, joints, and serosal surfaces) include antimalarials and MTX, which can be quite effective. In more severe cases, corticosteroids and immunosuppressive agents need to be used, despite the risk of potentially severe adverse effects. The need for more specific and less toxic treatments is obvious.
Some of the new medications described above have shown promise, and some have not, but there is much optimism for the future. With greater understanding and a little patience, we think that at least some of the newer agents will be integrated into routine clinical care in the years to come.
References:
References
1. Singh RR. SLE: translating lessons from model systems to human disease [published correction appears in Trends Immunol. 2006;27:59-60]. Trends Immunol. 2005;26:572-579.
2. Pitashny M, Schwartz N, Putterman C. Managing lupus: current and developing therapies. J Musculoskel Med. 2006;23:219-236.
3. Bertsias G, Ioannidis JP, Boletis J, et al; Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR recommendations for the treatment of systemic lupus erythematosus: Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195-205.
4. Hahn BH. Systemic lupus erythematosus. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s Principles of Internal Medicine. 16th ed. New York: McGraw-Hill; 2005:1965.
5. Haque S, Gordon C, Isenberg D, et al. Risk factors for clinical coronary heart disease in systemic lupus erythematosus: the lupus and atherosclerosis evaluation of risk (LASER) study. J Rheumatol. 2010;37:322-329.
6. Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363-369.
7. Rabbani MA, Habib HB, Ialam M, et al. Early renal damage assessed by the SLICC/ACR damage index is predictor of severe outcome in lupus patients in Pakistan. Lupus. 2010 Aug 12; [Epub ahead of print].
8. Aceves-Avila FJ, Benites-GodÃnez V. Drug allergies may be more frequent in systemic lupus erythematosus than in rheumatoid arthritis. J Clin Rheumatol. 2008;14:261-263.
9. Zonana-Nacach A, Barr SG, Magder LS, Petri M. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum. 2000;43:1801-1808.
10. Manzi S. Systemic lupus erythematosus: treatment. In: Kippel JH, Crofford L, Stone JH, Weyand CM, eds. Primer on the Rheumatic Diseases. Atlanta: The Arthritis Foundation; 2001:346-352.
11. Recommendation for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American College of Rheumatology ad hoc committee on glucocorticoid-induced osteoporosis. Arthritis Rheum. 2001;44:1496-1503.
12. Toubi E, Rosner I, Rozenbaum M, et al. The benefit of combining hydroxychloroquine with quinacrine in the treatment of SLE patients. Lupus. 2000;9:92-95.
13. Meinão IM, Sato EI, Andrade LE, et al. Controlled trial with chloroquine diphosphate in systemic lupus erythematosus. Lupus. 1996;5:237-241.
14. Tsakonas E, Joseph L, Esdaile JM, et al. A long-term study of hydroxychloroquine withdrawal on exacerbations in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. Lupus. 1998;7:80-85.
15. Fessler BJ, Alarcón GS, McGwin G Jr, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 2005;52:1473-1480.
16. Pons-Estel GJ, Alarcón GS, González LA, et al; LUMINA Study Group. Possible protective effect of hydroxychloroquine on delaying the occurrence of integument damage in lupus: LXXI, data from a multiethnic cohort. Arthritis Care Res (Hoboken). 2010;62:393-400.
17. Pons-Estel GJ, Alarcón GS, McGwin G, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum. 2009;61:830-839.
18. Shinjo SK, Bonfá E, Wojdyla D, et al; Grupo Latino Americano de Estudio del Lupus Eritematoso (GLADEL). Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum. 2010;62:855-862.
19. Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum. 1993;23(2 suppl 1):82-91.
20. Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823-835.
21. Hennessey EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293-307.
22. Lenert PS. Classification, mechanisms of action, and therapeutic applications of inhibitory oligonucleotides for Toll-like receptors (TLR) 7 and 9. Mediators Inflamm. 2010;2010:986596.
23. Tam LS, Gladman DD, Hallett DC, et al. Effect of antimalarial agents of the fasting lipid profile in systemic lupus erythematosus. J Rheumatol. 2000;27:2142-2145.
24. Borba EF, Bonfá E. Longterm beneficial effect of chloroquine diphosphate on lipoprotein profile in lupus patients with and without steroid therapy. J Rheumatol. 2001;28:780-785.
25. Lakshminarayanan S, Walsh S, Mohanraj M, Rothfield N. Factors associated with low bone mineral density in female patients with systemic lupus erythematosus. J Rheumatol. 2001;28:102-108.
26. Petri M. Hopkins Lupus Cohort: 1999 update. Rheum Dis Clin North Am. 2000;26:199-213, v.
27. Jung H, Bobba R, Su J. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010;62:863-868.
28. Broder A, Putterman C. The effects of hydroxychloroquine on antiphospholipid antibody titres in SLE. Ann Rheum Dis. 2010;69:A10-A11.
29. Carneiro JR, Sato EI. Double blind, randomized, placebo controlled clinical trial of methotrexate in systemic lupus erythematosus. J Rheumatol. 1999;26:1275-1279.
30. Fortin PR, Abrahamowicz M, Ferland D, et al; Canadian Network for Improved Outcomes in Systemic Lupus. Steroid-sparing effects of methotrexate in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2008;59:1796-1804.
31. Chan ES, Cronstein BN. Methotrexate-how does it really work? Nat Rev Rheumatol. 2010;6:175-178.
32. Contreras G, Pardo V, Leclercq B, et al. Sequential therapy for proliferative lupus nephritis. N Engl J Med. 2004;350:971-980.
33. Bernatsky S, Joseph L, Boivin JF, et al. The relationship between cancer and medication exposures in systemic lupus erythaematosus: a case-cohort study. Ann Rheum Dis. 2008;67:74-79.
34. Petri M. Cyclophosphamide: new approaches for systemic lupus erythematosus. Lupus. 2004;13:366-371.
35. Pisoni CN, Sanchez FJ, Karim Y, et al. Mycophenolate mofetil in systemic lupus erythematosus: efficacy and tolerability in 86 patients. J Rheumatol. 2005;32:1047-1052.
36. Somers EC, Marder W, Christman GM, et al. Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum. 2005;52:2761-2767.
37. Davis JC, Klippel JH. Anti-malarials and immunosuppressive therapies. In: Lahita RG, ed. Systemic Lupus Erythematosus. San Diego: Elsevier Academic Press; 2005:1273-1293.
38. Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121-2131.
39. D’Cruz DP, Houssiau FA. The Euro-Lupus Nephritis Trial: the development of the sequential treatment protocol. Lupus. 2009;18:875-877.
40. Appel GB, Contreras G, Dooley MA, et al; Aspreva Lupus Management Study Group. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103-1112.
41. Ginzler EM, Aranow C. Mycophenolate mofetil in lupus nephritis. Lupus. 2005;14:59-64.
42. Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med. 2000;343:1156-1162.
43. Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353:2219-2228.
44. Mak A, Cheak AA, Tan JY, et al. Mycophenolate mofetil is as efficacious as, but safer than, cyclophosphamide in the treatment of proliferative lupus nephritis: a meta-analysis and meta-regression. Rheumatology (Oxford). 2009;48:944-952.
45. Ginzler EM, Wofsy D, Isenberg D, et al; ALMS Group. Nonrenal disease activity following mycophenolate mofetil or intravenous cyclophosphamide as induction treatment for lupus nephritis: findings in a multicenter, prospective, randomized, open-label, parallel-group clinical trial. Arthritis Rheum. 2010;62:211-221.
46. Petri M, Jones RJ, Brodsky RA. High-dose cyclophosphamide without stem cell transplantation in systemic lupus erythematosus. Arthritis Rheum. 2003;48:166-173.
47. Burt RK, Traynor A, Statkute L, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295:527-535.
48. Otto MA. Stem-cell transplants can achieve durable remissions in severe SLE. Elsevier Global Medical News. July 27, 2010.
49. Sun L, Wang D, Liang J, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467-2475.
50. Thatayatikom A, White AJ. Rituximab: a promising therapy in systemic lupus erythematosus. Autoimmun Rev. 2006;5:18-24.
51. Lateef A, Petri M. Biologics in the treatment of systemic lupus erythematosus. Curr Opin Rheumatol. 2010;22:504-509.
52. Ramos-Casals M, Soto MJ, Cuadrado MJ, Khamashta MA. Rituximab in systemic lupus erythematosus: a systematic review of off-label use in 188 cases. Lupus. 2009;18:767-776.
53. Terrier B, Amoura Z, Ravaud P, et al; Club Rhumatismes et Inflammation. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum. 2010;62:2458-2466.
54. Conti F, Perricone C, Ceccarelli F, Valesini G. Rituximab treatment of systemic lupus erythematosus in controlled trials and in clinical practice: two sides of the same coin. Autoimmun Rev. 2010;9:716-720.
55. Dörner T, Kinnman N, Tak PP. Targeting B cells in immune-mediated inflammatory disease: a comprehensive review of mechanisms of action and identification of biomarkers. Pharmacol Ther. 2010;125;464-475.
56. Anolik JH, Barnard J, Cappione A, et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 2004;50:3580-3590.
57. Looney RJ, Anolik JH, Campbell D, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580-2589.
58. Saito K, Nawata M, Nakayamada S, et al. Successful treatment with anti-CD20 monoclonal antibody (rituximab) of life-threatening refractory systemic lupus erythematosus with renal and central nervous system involvement. Lupus. 2003;12:798-800.
59. Lee S, Ballow M. Monoclonal antibodies and fusion proteins and their complications: targeting B cells in autoimmune diseases. J Allergy Clin Immunol. 2010;125:814-820.
60. Davidson A, Diamond B, Wofsy D, Daikh D. Block and tackle: CTLA4Ig takes on lupus. Lupus. 2005;14:197-203.
61. Illiei GG, Shirota Y, Yarboro CH, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthitis Rheum. 2010;62:542-552.
62. Yao Y, Higgs BW, Richman L, et al. Use of type I interferon-inducible mRNAs as pharmacodynamic markers and potential diagnostic markers in trials with sifalimumab, an anti-IFNα antibody, in systemic lupus erythematosus. Arthritis Res Ther. 2010;12(suppl 1):S6.
63. Stohl W. A therapeutic role for BLyS antagonists. Lupus. 2004;13:317-322.
64. Navarra S, Guzman R, Gallacher A, et al. Belimumab,a BLys-specific inhibitor, reduced disease activity, flares and prednisone use in patients with active SLE: efficacy and safety results from the phase 3 BLISS-52 study. Arthritis Rheum. 2009;60(suppl 10):Abstract LB1.
65. van Vollenhoven RF, Zamani O, Wallace DJ, et al. Belimumab, a Bly specific inhibitor, reduces disease activity and severe flares in seropositive SLE patients: BLISS-76 study. EULAR 2010 Congress; June 16-19, 2010; Rome. Abstract OP0068.
66. Petri M, Furie R, Merrill J, et al. Four-year experience of belimumab, a BLyS-specific inhibitor, in systemic lupus erythematosus (SLE) patients. Arthritis Rheum. 2009;60(suppl 10):S774.