Managing stress fractures in athletes
The nature of fractures in athletics varies by the sport. This article describes a comprehensive approach to detection and management of the variety of fractures that can be incurred during play.
Stress fractures occur frequently in athletes and may lead to significant disability and loss of time from sports training and competition. Appropriate evaluation, diagnosis, and treatment are needed to return athletes to play as quickly and safely as possible.
In the diagnosis of stress fractures, the athlete's sport and sex and the location of symptoms should be considered. Stress fractures account for 2% to 15% of all injuries and up to 28% of all lower extremity injuries in patients seen in sports clinics.1,2 The annual incidence of stress fractures in athletes ranges from 1.9% to 21.1%.1,3,4 The lifetime incidence has been reported to be as high as 32% in dancers and almost 20% in figure skaters.5,6
In college athletes, the incidence is consistently highest in track and field athletes, specifically, distance runners.7-9 The incidence in female athletes may be twice that in male athletes who compete in the same sport5,8,10; the higher incidence of injuries in women is echoed in military reports.11
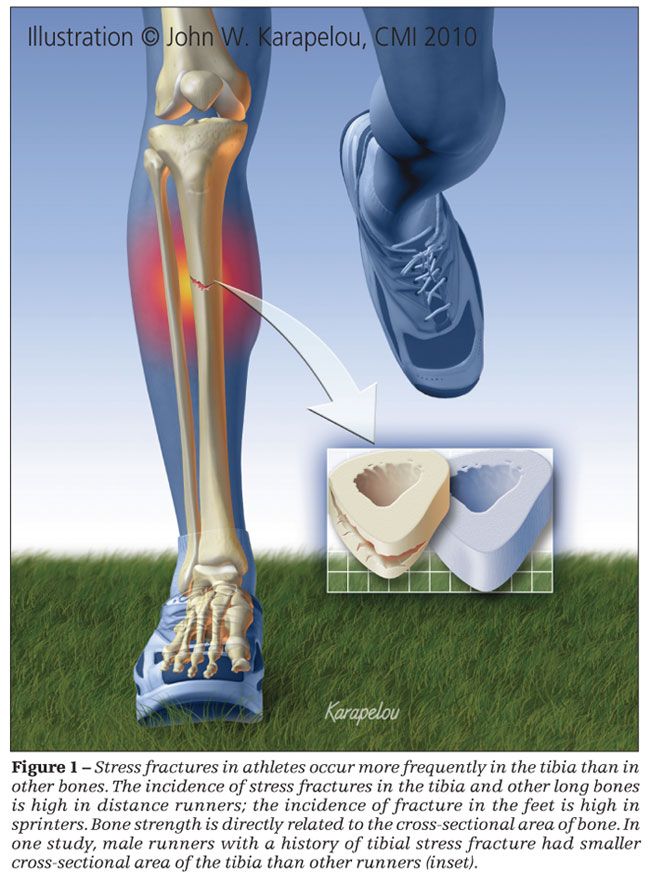
Overall, stress fractures occur most often in the bones of the foot. However, the single most frequently injured bone is the tibia.1,2,7,9,12 The anatomical site of a stress fracture varies by sport. Tennis, volleyball, soccer, and basketball players and distance runners have a higher incidence of stress fractures in the long bones, such as the tibia (Figure 1). Sprinters, hurdlers, jumpers, gymnasts, and skaters tend to have more stress fractures in the feet.1,12,13 Other sports are associated with stress fractures in non–lower extremity sites. For example, rowers are at increased risk for rib stress fractures and throwers for humeral and forearm stress fractures.14,15
Understanding of stress fracture epidemiology, risk factors, underlying causes, and assessment techniques is needed for effective diagnosis and treatment. In this article, we describe a comprehensive approach to evaluation and management of these injuries.
RISK FACTORS
Identifying and addressing risk factors for stress fractures in athletes may help prevent these injuries. Potential intrinsic and extrinsic factors include biomechanics, bone geometry, muscle mass and strength, changes in training regimens, menstrual function, and bone mineral density (BMD). Particular attention has been paid to identifying risk factors in female athletes because the incidence of injury is higher in these persons.
Lower extremity biomechanical factors, including leg, knee, and foot alignment, may contribute to stress fracture risk by leading to areas of stress concentration in bone or muscle when it is fatigued. In a prospective cohort study of track and field athletes, only leg-length discrepancy was found to increase the risk of stress fracture, and only in female athletes.1 A prospective study of military recruits demonstrated that those who had longer duration of foot pronation, as evaluated during treadmill walking before training, were at decreased risk for femoral neck and tibial stress fracture.16 In a cross-sectional study, foot and ankle pronation and pes cavus were associated with an increased risk of stress fracture, but knee and lower extremity alignment were not.12
A Cochrane database analysis found that use of shock-absorbing shoe inserts probably reduces stress fracture risk in military recruits. However, it was not able to identify the best design for such inserts or comment on risk in athletes because all studies included in the review involved military recruits.17
In the first study to evaluate acetabular retroversion, another potential biomechanical risk factor, Kuhn and associates18 demonstrated that military personnel experiencing femoral neck stress fractures have greater acetabular retroversion than uninjured controls. In athletes with recurring stress fractures, Korpelainen and associates19 found that pes cavus, leg-length inequality, and excessive forefoot varus are risk factors. Military studies evaluating biomechanical risk factors for recurring stress factors have produced inconsistent results. Because the available evidence is contradictory, no firm conclusions can be drawn regarding biomechanical factors and risk of stress fractures, except that leg-length discrepancy probably adds to stress fracture risk.
Because bone strength is directly related to the cross-sectional area of bone and the cross-sectional moment of inertia, bone geometry may contribute to the risk of stress fracture. Few studies have been performed that assess bone geometry in athletes. However, in studies that have measured components of bone geometry, a consistent relationship between bone geometry and stress fracture risk has been identified in male athletes and military recruits but not in females.
In a cross-sectional study of male runners in which CT was used to measure tibial geometry at the middle and distal third of the tibia, runners with a history of tibial stress fracture had smaller cross-sectional area, even after adjustments were made for body weight and height.20 A similar study in female athletes did not show a relationship between tibial cross-sectional area and stress fracture.21 Results of prospective studies in military populations consistently have shown a relationship between decreased cross-sectional area of the tibia and femur and increased risk of stress fracture in male recruits; study results for female recruits have been less consistent.22-24
Because muscles may dampen the forces exerted on bone during exercise, smaller muscle mass and muscle fatigue may play a role in the development of stress fractures by distributing more load to the bone.1 In female athletes, smaller calf girth was associated with lower extremity stress fractures.1 Military recruits with significantly decreased lower extremity strength, as measured by the leg press, had 5 times the risk of stress fracture compared with the recruits who had normal strength.25 In addition, muscle fatigue may result in alterations in running mechanics that could increase ground reactive forces exerted on bone.26
Improper training methods or changes in training could contribute to stress fracture risk by increasing the loading of bones and not allowing for adequate recovery time. Studies of these risk factors have shown conflicting results. In a study of ballet dancers, those who trained more than 5 hours a day were at 16 times greater risk for stress fracture than those who danced less than 5 hours a day.6 In a survey of competitive and recreational runners, Brunet and coworkers27 found that increased running mileage correlates with increased risk of stress fracture in women but not in men.
Cross-sectional studies have demonstrated that most runners with stress fracture can identify a change in their training routine before the occurrence of stress fracture.28 In addition, a study in the Israeli army demonstrated a significant decrease in stress fracture incidence with a decrease in cumulative marching during infantry training and enforcement of a minimum sleep regimen, thus reinforcing the importance of a proper training regimen that gradually increases training volume.24 The investigators noted that despite the change in training regimen, no difference in the soldiers' combat readiness was apparent, indicating that training volume can be decreased without affecting fitness outcomes. Other investigators have not noted an increased risk of stress fracture on the basis of differences in type or amount of training.1,19,29
Another small study found that treadmill runners may be at decreased risk for stress fracture compared with road runners because of less tibial strain seen with treadmill running. This finding indicates that training surface may have an influence on stress fracture risk.30
The athlete's fitness level also may play a role in stress fracture risk. Military studies have demonstrated that recruits who have lower levels of fitness are significantly more likely to experience stress fractures than their more fit counterparts.31
Stress fracture in female athletes may be a sign of the female athlete triad (disordered eating, amenorrhea, and low BMD). Therefore, researchers have investigated the roles of BMD, menstrual irregularity, and nutrition in stress fracture risk.
Low BMD or bone mineral content (BMC) may lead to reduced bone strength and contribute to the risk of stress fracture. A prospective study of 127 competitive female runners found that decreased whole-body BMC increases the risk of stress fracture.32 In a prospective study of female track and field athletes conducted by Bennell and colleagues,1 athletes who had stress fractures had lower BMC and lower BMD in the lumbar spine and feet than those who did not have stress fractures. Surprisingly, lower extremity BMD and total-body BMC were still higher in the women with stress fractures than in sedentary controls. The explanation for this is unknown; however, it has been suggested that the stress fractures are caused by a volume or intensity load beyond the inherent strength of the bone.
Other studies have shown that athletes with stress fractures may have decreased, increased, or normal BMD compared with controls.21,29 The cross-sectional nature of the studies and the timing and protocol of the bone density testing may account for differences in these results.
Investigators have demonstrated that menstrual history and dysfunction are predictive of decreased BMD and of stress fractures.1,6,33,34 Amenorrhea in athletes, which has been linked to decreased bone mass, probably results from a state of negative energy balance that is determined by the difference between caloric intake and energy expenditure.35 Initially, the mechanism of bone loss in these athletes was thought to be similar to that in postmenopausal women. However, investigators have proposed that decreased estrogen is not the only factor contributing to bone loss and that the energy deficiency observed in these athletes plays an important role.4
Some investigators have found that poor eating behaviors may be more prevalent in athletes who sustain stress fractures. In the study by Bennell and colleagues,1 athletes with stress fractures displayed more restrictive eating behaviors and scored higher on the Eating Attitudes Test, a screening tool designed to assess symptoms characteristic of eating disorders. Other studies have shown that female athletes who practice pathogenic weight control techniques are at significantly increased risk for stress fracture.36
In general, estimates of the prevalence of disordered eating in athletes range from 10% to 62%, varying with the athletic population evaluated and the definitions used for disordered eating.37-40 Elite athletes and athletes involved in sports that emphasize leanness are at increased risk for disordered eating.39
Poor dietary habits also may contribute to the risk of stress fracture; key nutrients needed to sustain normal bone growth and remodeling (eg, calcium) may be lacking. The currently available calcium intake research does not show a clear link between dietary calcium and stress fracture risk.41 However, a recent prospective evaluation of 125 competitive distance runners demonstrated a decreased risk of stress fracture in runners with higher intakes of calcium, skim milk, and dairy products.42 It was also noted that higher intakes of milk, dairy foods, animal protein, and potassium are associated with greater gains in whole-body BMD and BMC.
The study conducted by Myburgh and colleagues29 also found that athletes with stress fractures take in less calcium than controls. The groups did not differ in protein, fiber, alcohol, caffeine, vitamin D, or phosphorus intake. More investigations are needed to determine the relationship between nutrition and stress fractures. Acute weight loss also may be a risk factor.
DIAGNOSIS
History and physical examination
Usually, patients with stress fracture report activity-related pain that worsened gradually over time. Initially, pain often is noted only during athletic activity, but with continued activity, pain persists during rest.
Typically, physical examination reveals localized pain with palpation at the site of the stress fracture. Edema may be noted in the area of fracture. The patient may have pain with percussion distal to the site of fracture or when a tuning fork is placed over the site. Pain also may be elicited at the fracture site with the hop test (the patient hops on a single leg; exquisite pain suggests a stress fracture) or fulcrum test (the examiner places 1 arm under the sitting patient's femur and the other hand over the patient's knee and applies firm pressure upward on the femur and downward on the knee; exquisite pain suugests femur fracture).
Imaging
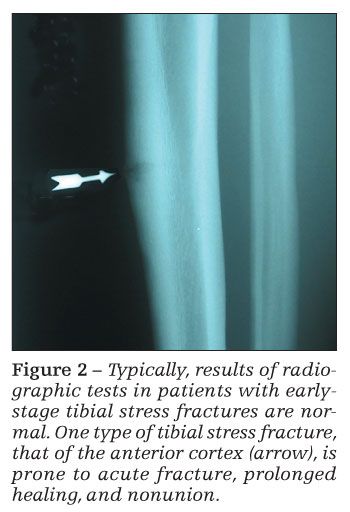
Results of radiographic tests may be normal in the initial stages of a stress fracture (Figure 2). In the later stages, periosteal reaction or a fracture line may be visualized. Plain x-ray films may have sensitivities as low as 15% early in the clinical course; with repeated radiographs in the later stages, sensitivity may be only about 50%.12,43
In 3-phase bone scintigraphy, acute stress fractures have abnormal uptake in all 3 phases of the bone scan; soft tissue injuries have uptake in the first 2 phases. Bone scintigraphy has been used widely and was long regarded the gold standard in stress fracture diagnosis. However, false-negative bone scan results have been reported in both the femoral neck and tibia in patients in whom the diagnosis was made with MRI.44,45
T1-weighted MRI with short tau inversion recovery sequences and T2-weighted MRI have been useful in the diagnosis of stress fracture. When fat-suppressed images are used, sensitivity and specificity are high and intraobserver and interobserver agreement are strong.46,47
Many investigators have found MRI to be at least as sensitive as bone scanning and more specific.48-50 Even early stress changes, such as bone marrow edema, may be visualized with MRI. In addition, soft tissue injuries (eg, periosteal edema, muscle and tendon injury, and osteonecrosis) may be diagnosed.47 When radiographic test results are normal and suspicion of a stress fracture is high, MRI is the fastest and least invasive method of diagnosing a stress fracture.
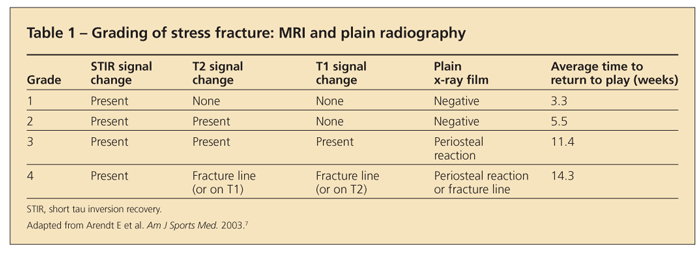
Systems have been constructed for grading stress injury with the use of MRI.51 Arendt and colleagues7 proposed a grading system that helps predict time to return to play (Table 1). Higher grades indicate longer recovery times.
Laboratory testing
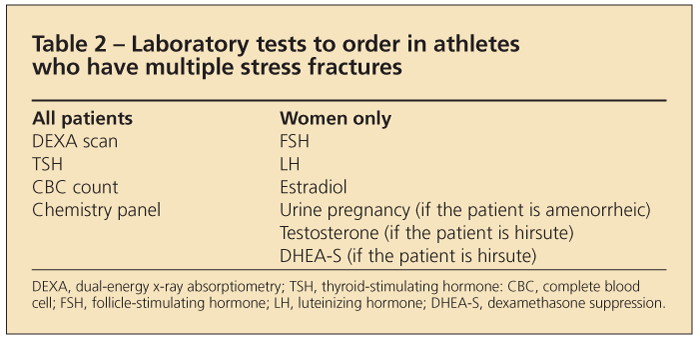
Athletes who have multiple stress fractures may require additional workup, including laboratory testing. Various tests are listed in Table 2.
TREATMENT
Regardless of the site of stress fracture, early treatment should include rest and avoidance of all exacerbating activities. Athletes should be encouraged to maintain fitness by engaging in nonpainful activities, such as bicycling, swimming, and pool-running. Some athletes-those who have high-risk stress fractures or pain with activities of daily living-may require complete non–weight bearing with crutches, bracing, or casting to allow for healing and relieve pain.
This period of rest should be followed by a gradual return to activity. Institutions use various protocols in guiding return to play. There is no literature that compares them, but there is a fairly standard protocol: as athletes become asymptomatic with various levels of participation, they are allowed to progress gradually from pain-free walking to participation in low-impact weight-bearing activities to participation in sport-specific activities to participation in unrestricted play.
Tibial stress fractures may be difficult to manage because nonunions may develop, and they may require surgery. The use of pneumatic leg braces significantly hastens the return to pain-free sports participation.52,53 Swenson and coworkers54 found that braced athletes could return to light activity about 14 days earlier than unbraced athletes and to full unrestricted activities about 50 days earlier.
Stress fractures of the anterior cortex of the tibia are at greater risk for acute fracture, prolonged healing, and nonunion.55 These fractures often require surgical intervention with an intramedullary rod.
Other common stress fractures include tarsal navicular and femoral neck stress fractures. Patients with stress fractures of the tarsal navicular bone require significant periods of non–weight-bearing immobilization to allow for proper healing. Typically, athletes with navicular stress fractures need 4 to 6 weeks of immobilization in a non–weight-bearing cast.
Femoral neck stress fractures, particularly those in the superior cortex, are associated with multiple complications, such as osteonecrosis of the femoral head, delayed union, and nonunion. Stress fractures on the tension side of the neck indicate a high risk of complete fracture. Patients with these fractures need careful monitoring and often require surgical fixation.55
Special consideration should be given to female athletes who have stress fracture and an additional risk factor for the female athlete triad, such as disordered eating, amenorrhea, or recurring stress fracture. In amenorrheic athletes, restoration of normal menstrual function is important. In treatment of patients with menstrual dysfunction and decreased BMD, the usefulness of estrogen replacement is unclear. An association between improved BMD and estrogen use was shown in early cross-sectional investigations.29,34
A small positive effect on BMD with oral contraceptive pill use was seen in some prospective studies, but weight gain was found to be even more effective than estrogen therapy in all these studies.56,57 More recent prospective and cross-sectional data suggest that contraceptive pill use has either no effect or a negative effect on BMD.
Again, participants who gained weight during these studies had significant BMD gains.40,58 This supports the theory that estrogen deficiency is not the sole cause of decreased BMD in these athletes and that the caloric deficiency that is found in them is more important.4 Therefore, nutritional counseling should be provided for these athletes, and the importance of proper energy intake should be emphasized.
Hilton and Loucks59 found that improved nutritional status leads to the return of normal hormonal function, even with continued exercise. In several case studies, a program of diet supplementation and moderately decreasing training frequency was found to allow for weight gain, eventual return of menstrual function, and accompanying improvement in athletic performance.60,61
Drinkwater and colleagues62 found that calcitonin-salmon nasal spray improves BMD in the proximal femur and spine in amenorrheic athletes. Other medications that are useful in the treatment of postmenopausal women with osteoporosis, such as bisphosphonates and selective estrogen receptor modulators, are not approved for use in amenorrheic women of childbearing age. These medications have not been well studied, and they may have teratogenic effects. If dietary sources are not sufficient (which is often the case), athletes who have amenorrhea and decreased BMD should be provided with calcium and vitamin D supplementation.39,63
References:
References
1. Bennell KL, Malcolm SA, Thomas SA, et al. The incidence and distribution of stress fractures in competitive track and field athletes: a twelve-month prospective study. Am J Sports Med. 1996;24:211-217.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8:273-278.
3. Goldberg G, Pecora C. Stress fractures: a risk of training in the freshman. Phys Sportsmed. 1994;22:68-78.
4. Zernicke R, McNitt-Gray J, Otis C, et al. Stress fracture risk assessment among elite collegiate women runners. J Biomech. 1994;27:854.
5. Dubravcic-Simunjak S, Pecina M, Kuipers H, et al. The incidence of injuries in elite junior figure skaters. Am J Sports Med. 2003;31:511-517.
6. Kadel NJ, Teitz CC, Kronmal RA. Stress fractures in ballet dancers. Am J Sports Med. 1992;20:445-449.
7. Arendt E, Agel J, Heikes C, Griffiths H. Stress injuries to bone in college athletes: a retrospective review of experience at a single institution. Am J Sports Med. 2003;31:959-968.
8. Hame SL, LaFemina JM, McAllister DR, et al. Fractures in the collegiate athlete. Am J Sports Med. 2004;32: 446-451.
9. Tran J, Hame S, Maguire R, McAllister D. Fractures in track and field athletes: a 12 year study. Med Sci Sports Exerc. 2004;36:S324.
10. Nattiv A. Female track athletes are at increased risk for stress fractures. Med Sci Sports Exerc. 2002;34:S157.
11. Friedl KE, Nuovo JA, Patience TH, Dettori JR. Factors associated with stress fracture in young army women: indications for further research. Mil Med. 1992;157:334-338.
12. Matheson GO, Clement DB, McKenzie DC, et al. Stress fractures in athletes: a study of 320 cases. Am J Sports Med. 1987;15:46-58.
13. Ohta-Fukushima M, Mutoh Y, Takasugi S, et al. Characteristics of stress fractures in young athletes under 20 years. J Sports Med Phys Fitness. 2002;42:198-206.
14. Warden SJ, Gutschlag FR, Wajswelner H, Crossley KM. Aetiology of rib stress fractures in rowers. Sports Med. 2002;32:819-836.
15. Sabick MB, Torry MR, Kim YK, Hawkins RJ. Humeral torque in professional baseball pitchers. Am J Sports Med. 2004;32:892-898.
16. Hetsroni I, Finestone A, Milgrom C, et al. The role of foot pronation in the develoment of femoral and tibial stress fractures: a prospective biomechanical study. Clin J Sport Med. 2008;18:18-23.
17. Rome K, Handoll HH, Ashford RL. Interventions for preventing and treating stress fractures and stress reactions of bone of the lower limbs in young adults. Cochrane Database Syst Rev. 2005;(2):CD000450.
18. Kuhn KM, Riccio AI, Saldua NS, Cassidy J. Acetabular retroversion in military recruits with femoral neck stress fractures. Clin Orthop Relat Res. 2010;468:846-851.
19. Korpelainen R, Orava S, Karpakka J, et al. Risk factors for recurrent stress fractures in athletes. Am J Sports Med. 2001;29:304-310.
20. Crossley K, Bennell KL, Wrigley T, Oakes BW. Ground reaction forces, bone characteristics, and tibial stress fracture in male runners. Med Sci Sports Exerc. 1999;31:1088-1093.
21. Bennell K, Crossley K, Jayarajan J, et al. Ground reaction forces and bone parameters in females with tibial stress fracture. Med Sci Sports Exerc. 2004;36:397-404.
22. Beck TJ, Ruff CB, Mourtada FA, et al. Dual-energy x-ray absorptiometry derived structural geometry for stress fracture prediction in male U.S. Marine Corps recruits. J Bone Miner Res. 1996;11:645-653.
23. Giladi M, Milgrom C, Simkin A, et al. Stress fracture and tibial bone width: a risk factor. J Bone Joint Surg. 1987;69B:326-329.
24. Finestone A, Milgrom C. How stress fracture incidence was lowered in the Israeli army: a 25-yr struggle. Med Sci Sports Exerc. 2008;40(11 suppl):S623-S629.
25. Hoffman JR, Chapnik L, Shamis A, et al. The effect of leg strength on the incidence of lower extremity overuse injuries during military training. Mil Med. 1999;164:153-156.
26. Nyland JA, Shapiro R, Stine RL, et al. Relationship of fatigued run and rapid stop to ground reaction forces, lower extremity kinematics, and muscle activation. J Orthop Sports Phys Ther. 1994;20:132-137.
27. Brunet ME, Cook SD, Brinker MR. A survey of running injuries in 1505 competitive and recreational runners. J Sports Med Phys Fitness. 1990;30:307-315.
28. Ferry AT, Graves T, Theodore GH, Gill TJ. Stress fractures in athletes. Phys Sportsmed. 2010;38:109-116.
29. Myburgh KH, Hutchins J, Fataar AB, et al. Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med. 1990;113:754-759.
30. Milgrom C, Finestone A, Segev S, et al. Are overground or treadmill runners more likely to sustain tibial stress fracture? Br J Sports Med. 2003;37:160-163.
31. Beck TJ, Ruff CB, Shaffer RA, et al. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000;27:437-444.
32. Kelsey JL, Bachrach LK, Procter-Gray E, et al. Risk factors for stress fracture among young female cross-country runners. Med Sci Sports Exerc. 2007;39:1457-1463.
33. Barrow G, Saha S. Menstrual irregularity and stress fractures in collegiate female distance runners. Am J Sports Med. 1988;16:209-216.
34. Lloyd T, Triantafyllou SJ, Baker ER, et al. Menstrual function and bone mass in elite women distance runners: endocrine and metabolic features. Ann Intern Med. 1986;102:158-163.
35. Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J App Physiol. 1998;84:37-46.
36. Nattiv A, Puffer JC, Green GA. Lifestyles and health risks of collegiate athletes: a multi-center study. Clin J Sport Med. 1997;7:262-272.
37. Marshall JD, Harber VJ. Body dissatisfaction and drive for thinness in high performance field hockey athletes. Int J Sports Med. 1996;17:541-544.
38. O'Connor PJ, Lewis RD, Kirchner EM. Eating disorder symptoms in female college gymnasts. Med Sci Sports Exerc. 1995;27:550-555.
39. Sundgot-Borgen J, Larsen S. Preoccupation with weight and menstrual function in female elite athletes. Scand J Med Sci Sports Exerc. 1993;3:156-163.
40. Warren B, Stanton A, Blessing D. Disordered eating patterns in competitive female athletes. Int J Eat Disord. 1990;9:565-569.
41. Cline AD, Jansen GR, Melby CL. Stress fractures in female army recruits: implications of bone density, calcium intake, and exercise. J Am Coll Nutr. 1998;17:128-135.
42. Nieves JW, Melsop K, Curtis M, et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PMR. 2010;2:740-750.
43. Anderson MW, Ugalde V, Batt M, Gacayan J. Shin splints: MR appearance in a preliminary study. Radiology. 1997;204:177-180.
44. Milgrom C, Chisin R, Giladi M, et al. Negative bone scans in impending tibial stress fractures: a report of three cases. Am J Sports Med. 1984;12:488-491.
45. Wen DY, Propeck T, Singh A. Femoral neck stress injury with negative bone scan. J Am Board Fam Pract. 2003;16:170-174.
46. Ahovuo J, Kiuru M, Kinnunen J, et al. MR imaging of fatigue stress injuries to bones: intra- and interobserver agreement. Magn Reson Imaging. 2002;20:401-406.
47. Aoki Y, Yasuda K, Tohyama H, et al. Magnetic resonance imaging in stress fractures and shin splints. Clin Orthop Relat Res. 2004;421:260-267.
48. Boniotti V, Del Giudice E, Fengoni E, et al. Imaging of bone micro-injuries. Radiol Med (Torino). 2003;105:425-435.
49. Kiuru M, Pihlajamaki H, Hietanen HJ, Ahovuo J. MR imaging, bone scintigraphy, and radiography in bone stress injuries of the pelvis and the lower extremity. Acta Radiol. 2002;43:207-212.
50. Shin AY, Morin WD, Gorman JD, et al. The superiority of magnetic resonance imaging in differentiation the cause of hip pain in endurance athletes. Am J Sports Med. 1996;24:168-176.
51. Fredericson M, Bergman AG, Hoffman KL, Dillingham MS. Tibial stress reactions in runners: correlation of clinical symptoms and scintigraphy with a new magnetic resonance imaging grading system. Am J Sports Med. 1995;23:472-481.
52. Dickson TB Jr, Kichline PD. Functional management of stress fracture in female athletes using a pneumatic leg brace. Am J Sports Med. 1987;15:86-89.
53. Whitelaw GP, Wetzler MJ, Levy AS, et al. A pneumatic leg brace for the treatment of tibial stress fractures. Clin Orthop Relat Res. 1991;270:301-305.
54. Swenson EJ Jr, DeHaven KE, Sebastianelli WJ, et al. The effect of a pneumatic leg brace on return to play in athletes with tibial stress fractures. Am J Sports Med. 1997;25:322-328.
55. Orava S, Hulkko A. Delayed unions and nonunions of stress fractures in athletes. Am J Sports Med. 1988;16:378-382.
56. Gulekli B, Davies MC, Jacobs HS. Effect of treatment on established osteoporosis in young women with amenorrhea. Clin Endocrinol. 1994;41:275-281.
57. Haenggi W, Casez JP, Birkhaeuser MH, et al. Bone mineral density in young women with long-standing amenorrhea: limited effect of hormone replacement therapy with ethinylestradiol and desogestrel. Osteoporos Int. 1994;4:99-103.
58. Polatti F, Perotti F, Filippa N, et al. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221-224.
59. Hilton LK, Loucks AB. Low energy availability, not exercise stress, suppresses the diurnal rhythm of leptin in healthy young women. Am J Physiol Endocrinol Metab. 2002;278:E43-E49.
60. Dueck CA, Matt KS, Manore MM, Skinner JS. Treatment of athletic amenorrhea with a diet and training intervention program. Int J Sports Nutr. 1996;6:24-40.
61. Kopp-Woodroffe SA, Manore MM, Dueck CA, et al. Energy and nutrient status of amenorrheic athletes participating in a diet and exercise training intervention program. Int J Sport Nutr. 1999;9:70-88.
62. Drinkwater BL, Healy NL, Rencken ML, et al. Effectiveness of nasal calcitonin in preventing bone loss in young amenorrheic women. J Bone Miner Res. 1993;8:S264.
63. Kirchner EM, Lewis RD, O'Connor PJ. Bone mineral density and dietary intake of female college gymnasts. Med Sci Sports Exerc. 1995;27:543-549.