Secukinumab Improves Spinal Pain in Patients With Axial Spondyloarthritis
Chronic back pain, a common symptom of axial spondyloarthritis that increases disease burden and reduces quality of life, may be improved by subcutaneous injection of secukinumab 150 mg or 300 mg.
Secukinumab quickly and significantly improved spinal pain in patients with axial spondyloarthritis (axSpA) at both Week 8 and up to Week 24, according to a study published in Therapeutic Advances in Musculoskeletal Disease.1
“Chronic back pain is the cardinal symptom of axSpA and, although multifactorial, is related to inflammation in the sacroiliac joints and in the spine. Inflammation is followed by repair and new bone formation, resulting in spinal ankylosis that is associated with mobility restrictions in the axial skeleton,” investigators explained. “This contributes to increased disease burden and limits the performance of daily activities in individuals with axSpA.”
In SKIPPAIN, a double-blind, placebo-controlled, multicenter study, investigators randomized patients 3:1 to receive either secukinumab 150 mg via subcutaneous injection (Group A) or placebo (Group B) during treatment period 1 (TP1; baseline to Week 8). In TP2 (Week 8 through Week 24), patients were assigned to 1 of 5 treatment arms: Arms A1, A2, A3, B1, and B2 (see infographic).
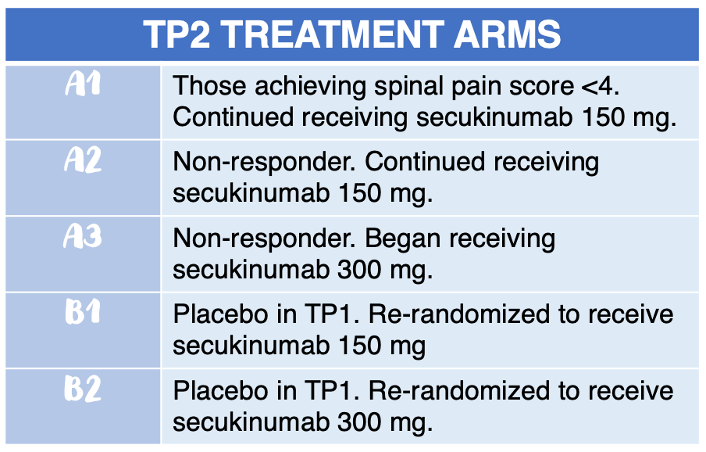
Eligible patients were aged 18 years or older, had an axSpA diagnosis, active spinal disease of ≥4 defined by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and average spinal pain score of >4. Patients were also expected to have an inadequate response to at least 2 non-steroidal anti-inflammatory drugs (NSAIDs) for 4 or more weeks.
The primary endpoint was to determine efficacy of secukinumab 150 mg in achieving an average spinal pain score of <4 by Week 8. The secondary endpoint was to analyze improvement in BASDAI scores (<4) at Week 8 for those receiving secukinumab 150 mg. Safety was assessed by the number of adverse events (AEs), serious adverse events (SAEs), and any AEs of note in both TP1 and TP2.
Of the 380 patients included in the study, 285 patients were initially placed in the secukinumab 150 mg cohort, and 95 patients were placed in the placebo group.
As early as Week 1, responder rates were higher in the secukinumab 150 mg cohort when compared with placebo (9.8% vs 2.1%, respectively), a trend which continued to Week 8 (31.9% vs 20.0%, respectively). Response to average spinal pain scores was higher in the secukinumab 150 mg group when compared with placebo (OR: 1.89; 95% CI: 1.08–3.33; p = 0.0264). Additionally, nocturnal spinal pain scores were significantly better in the secukinumab 150 mg group (OR: 2.38; 95% CI: 1.31–4.31; p = 0.0043). However, total spine pain scores were comparable (OR: 1.72; 95% CI: 0.95–3.10; p = 0.0720).
At the 8-Week mark, 33.3% of patients receiving secukinumab 150 mg achieved a BASDAI score of <4, compared with only 23.2% in the placebo cohort. ASDAS scores also improved in the secukinumab 150 mg group when compared with placebo (3.75–2.52 vs 3.67–3.19).
During TP2, improvements in pain scores were the highest in the secukinumab 150 mg (Arm A1) for average pain score (1.77), total pain score (2.00), and nocturnal pain score (1.53) when compared with the other cohorts. BASDI improved the most for patients in Arm A1, followed by Arm A2 (-1.53), and Arm A3 (-1.62). Similarly, ASDAS reductions (improvements) were highest in Arm A1, Arm A2 (-1.24), and Arm A3 (-1.47). In Arm B1 and B2, BASDAI reductions from Week 8 were -2.06 and -2.23, respectively. ASDAS reductions from baseline were -1.49 and -1.77, respectively.
Assessment of SpondyloArthritis international Society health index (ASAS-HI) and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scores improved among all treatment arms from baseline to Week 24, with Arm A1 experiencing the highest average improvement (-5.2 and 16.48, respectively).
Only 2.4% (n = 9) patients receiving secukinumab and/or placebo reported SAEs during the treatment period. The most common AEs across all groups were headache, nasopharyngitis, oropharyngeal pain, and upper respiratory tract infection.
Investigators failed to come to a consensus on the threshold of 4 as the primary endpoint for spine pain response, which limited the study. Additionally, depression and psychological distress, which may impact disease perception, were not analyzed during the study period. Lastly, up-titration at Week 8 may not have been ideal as improvements were seen up to Week 24 in the secukinumab 150 mg cohort.
“The SKIPPAIN trial demonstrated that secukinumab provided a rapid and significant improvement in spinal pain and reduced disease activity in patients with axSpA,” investigators concluded. “The up-titration strategy might be beneficial for sub-optimal responders to secukinumab 150 mg. ‘Residual’ pain might still be an issue and a reason for some patients not achieving the treatment goal, warranting further investigation.”
Reference:
Poddubnyy, D., et al.,2021. Rapid improvement in spinal pain in patients with axial spondyloarthritis treated with secukinumab: primary results from a randomized controlled phase-IIIb trial. [online] Therapeutic Advances in Musculoskeletal Disease. Available at: <https://journals.sagepub.com/doi/full/10.1177/1759720X211051471#> [Accessed 26 October 2021].