Osteoarthritis Diagnosis: Avoiding the Pitfalls
A common pitfall in the diagnosis of osteoarthritis (OA) is misinterpretation of the patient's symptoms and signs.
Correct diagnosis of osteoarthritis (OA) is important-misdiagnosis often leads to omission of appropriate treatment or institution of unnecessary treatment. It also may create stress for the patient.
A common pitfall in the diagnosis of OA is misinterpretation of the patient's symptoms and signs. Pain is the predominant symptom in patients with OA, and OA pain has typical characteristics. However, pain may arise not only from intra-articular structures but also from periarticular muscle spasm or soft tissue rheumatism. Differentiation of articular pain from periarticular pain is important because the latter often may be managed successfully by local injection of a depot corticosteroid preparation and physical therapy, without systemic medication.
In this 3-part article, I review the pathogenesis, diagnosis, and management of OA. The first part (“Defining osteoarthritis: What it is, and what it is not,” The Journal of Musculoskeletal Medicine, September 2010, page 338) offered a contemporary, operational, evidence-based definition of the disease that has evolved from growing knowledge of its cause. In this second part, I discuss the clinical aspects of OA, including diagnosis and the diagnostic pitfalls. The third part, to appear in an upcoming issue of this journal, will provide an overview of current approaches to treatment.
THE CLINICAL PICTURE
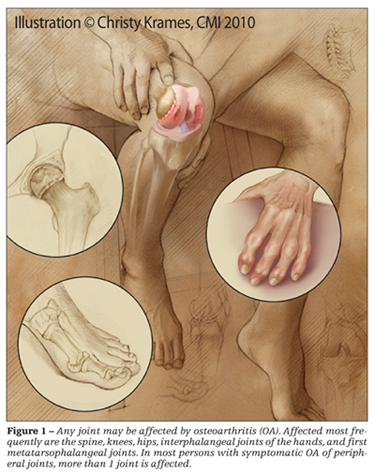
OA can affect any joint in the body; those most frequently affected are the spine, knees, hips, interphalangeal joints of the hands, and first metatarsophalangeal (MTP) joints (Figure 1). In most persons with symptomatic OA of peripheral joints, more than 1 joint is affected. Of 500 patients with symptomatic OA in peripheral joints, only 6% had symptoms confined to a single joint.1 The most frequently involved joints were the knees (41% of affected joints), hands (30%), and hips (19%).
In patients with hip OA, there may be a congenital or developmental abnormality of that joint (eg, Legg-Calv-Perthes disease [osteonecrosis of the secondary center of ossification in the femoral head], slipped femoral capital epiphysis, congenital dysplasia). In contrast, congenital or developmental abnormalities seldom are a basis for knee OA, in which a history of previous trauma, meniscectomy, obesity, and some repetitive vocational activities are dominant risk factors. Spine OA, which most often affects the lumbar and cervical regions, typically is not associated with a history of trauma. Although OA may involve any diarthrodial joint in the body, in the absence of trauma or of a developmental or congenital abnormality, it is uncommon in the elbows, glenohumeral joints, ankles, and wrists.
No one would be surprised if OA developed in a knee with hyperextension and varus angulation resulting from catastrophic acute trauma, such as a vigorous below-the-knee tackle of a football player, which disrupts the integrity of the joint by damaging ligaments, articular cartilage, the meniscus, and subchondral bone. However, on the basis of experience with OA in patients presenting to lower extremity adult reconstructive clinics in their institution, Brown and associates2 estimated that roughly 12% of the overall prevalence of symptomatic OA is attributable to posttraumatic OA (1.6%, 9.8%, and 20.5% of the totality of hip, knee, and ankle OA, respectively); that is, “posttraumatic” OA accounted for only a minority of cases of clinical OA.
Symptoms: What are the
patient's complaints?

In the vast majority of cases, the clinical feature that leads patients with OA to seek medical attention is joint pain (Table 1). Systemic manifestations (eg, fever, anemia, weight loss) are not features of primary OA (Table 2).
Typically, the discomfort has been present for months or years and has been only slowly progressive. The pain often is described as a deep, dull ache, localized to the involved joint, aggravated by use of that joint-especially by weight bearing in the case of knee or hip OA-and relieved by rest. Although the pain initially is intermittent, with disease progression it may become constant, increasingly severe, and disabling.
Nocturnal pain that interferes with sleep is seen mostly in advanced hip OA, in which the pain often is associated with an effusion of the joint. The presence of night pain in patients with hip OA was found to predict the presence of a hip joint effusion at the time of hip arthroplasty with greater specificity than ultrasonography (94% vs 70%), although with slightly lower sensitivity (92% vs 85%).3
In patients with hip OA, movements that require internal rotation are particularly likely to evoke

pain. In those with knee OA, extremes of flexion (eg, squatting) or of extension may be painful. In those with OA of the thumb base or other joints of the hand, pinch movements and tight grasp, as required for opening a jar, may lead to discomfort.
In some patients with OA, pain may be referred. For example, pain resulting from hip OA may be referred to the knee. In patients with cervical spine OA, it may be localized to the shoulder, arm, forearm, or hand; in some cases, pain at these sites may occur in the absence of neck pain.
Pain resulting from OA of the cervical or lumbar spine may have a radicular component (ie, it may be sharp and shooting and aggravated by the increase in intrathecal pressure generated by coughing, sneezing, or straining with a bowel movement). Physical examination may reveal neurological signs, such as altered sensation, reduced strength, and diminution of a deep tendon reflex, consistent with involvement of a single nerve root.
Stiffness, crepitus,
deformity, limp
In addition to joint pain, patients with OA may complain of joint stiffness. Although morning stiffness is a classic feature of more inflammatory joint disease (eg, rheumatoid arthritis [RA]), it is less well recognized as a feature of OA. The morning stiffness of RA may persist for hours after the patient awakens, but in OA, morning stiffness usually lasts no longer than 20 to 30 minutes. This “gelling” sensation in patients with OA may be prominent not only on arising but also after any period of inactivity, such as an automobile ride or an evening in a theater seat.
The stiffness induced by inactivity during the day, like morning stiffness, usually subsides rapidly. In patients with knee involvement, it typically abates after only a few steps. With progression of the disease, however, the stiffness becomes more prolonged.
Patients with OA often complain of crepitus, the sensation of “cracking” or “popping” of tissues of the involved joint rubbing against each other with movement. Often, the crepitus in OA is audible. It is most common in patients with knee OA.
Deformity is a complaint in some patients with OA. They may note, for example, that one knee has become larger than the other or that the base of the big toe or a distal interphalangeal (DIP) joint of the hand has become enlarged. They also may complain of angular deformities, such as bowing of the knees (varus) resulting from OA of the medial tibiofemoral compartment or squaring of the thumb base resulting from involvement of the first carpometacarpal joint or metacarpotrapezial joint.
In patients with hip or knee OA, development of a limp may be a major source of concern, especially when the disturbance is noticeable to others as they watch the patient walk. Walking on an uneven surface or on a ramp tends to exaggerate the limp.
Origins of joint pain in OA
Because articular cartilage is aneural, the joint pain in OA must arise from other structures. In some cases, it may be the result of stretching of nerve endings in the periosteum covering osteophytes. In others, it may arise from synovitis or from microfractures in subchondral bone.
In still others, the joint pain in OA may represent bone angina caused by distortion of medullary blood flow by the thickened subchondral trabeculae, which may increase the intraosseous pressure and cause severe intraosseous stasis.4,5 This hemodynamic abnormality is reflected in a prolongation of the emptying time after intraosseous injection of radiopaque contrast material into the femoral neck. Joint instability (leading to stretching of the joint capsule, muscle spasm, enthesopathy, and bursitis) is an additional source of pain. Like the medullary hypertension, which results from the response of the subchondral bone to mechanically induced damage, synovitis is a common cause of joint pain in OA. It is caused by soluble and particulate breakdown products of the damaged articular cartilage and subchondral bone.
The joint pain of OA may arise from periarticular as well as articular structures. Common in patients with OA are soft tissue rheumatism in areas adjacent to the involved joint (eg, anserine bursitis in patients with knee OA), trochanteric bursitis in patients with hip OA, and subacromial bursitis or bicipital tendinitis in patients with glenohumeral or subacromial joint OA.
Physical examination of a joint with OA may reveal tenderness and bony or soft tissue swelling (see Table 1). Crepitus, which may be diffuse or localized to marginal osteophytes or to the synovium, is characteristic of OA. It may present as the soft crepitus of fibrillated cartilage, as in the patellofemoral joint, or the harder, sharp crepitus of joints in which articular cartilage has been lost so that adjacent bony surfaces rub against each other with movement. The latter is particularly noticeable in OA of the thumb base and of the knee.
Synovial effusions, when present, usually are not large, although those in the knee or shoulder may occasionally be voluminous. Palpation may reveal some warmth over the joint.
In the advanced stages of OA, gross deformity, palpable bony hypertrophy, subluxation, and marked loss of joint motion, often with noticeable contracture, may be striking. Enlargement of the joint may be the result of effusion, synovial thickening, or osteophytes, which can alter the contour of the joint significantly.
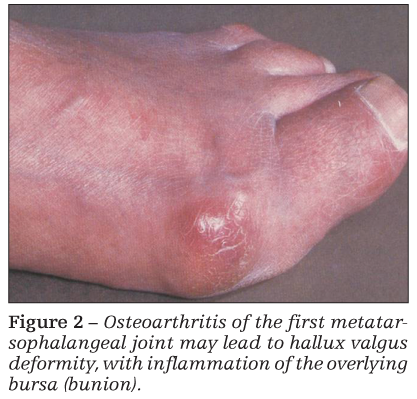
Bony swelling resulting from osteophytes is particularly noticeable in patients with Heberden or Bouchard nodes (interphalangeal joint OA of the hands) and OA of the first MTP joint (bunion) (Figure 2). Atrophy of periarticular muscle may be caused by disuse (as a result of unloading of the painful extremity) and may exaggerate the appearance of joint swelling.
Clinical course of OA
The common view that once OA becomes symptomatic it follows an inexorably progressive and downhill course is inaccurate. Physicians might help their patients by letting them know that the disease does not worsen in all patients, that it stabilizes in many, and that in some, especially those with knee OA, regression of joint pain and even of radiographic changes may occur.
Massardo and colleagues6 conducted an early study of patients with knee OA who underwent clinical and radiographic evaluation on 2 occasions 8 years apart. Although 20% of the patients worsened over the interval and many incurred severe disability, 13% improved and 2 patients showed striking improvement in function.
In another study, only 33% of patients for whom paired knee radiographs were obtained at a mean interval of 11 years showed radiographic progression.7 Notably, pain scores also tended to not worsen. Thus, it may be concluded that many patients with knee OA do not deteriorate radiographically or symptomatically over lengthy periods of observation.
However, it is important to identify the subset of patients who undergo more rapid disease progression and to direct early intervention efforts toward that high-risk group. In this context, note that although loss of articular cartilage is the predominant pathological feature of OA, in standing knee x-ray films, the radiographic diagnosis of OA often is made on the basis of osteophytosis alone-without joint-space narrowing. However, in the absence of joint-space narrowing or other bony change, osteophytes seen in knee x-ray films may be the result of aging alone, and not of progressive OA.8
Guidelines for a clinical
diagnosis of OA
A multidisciplinary guideline development group representing 12 European countries within the European League Against Rheumatism (EULAR) recently provided recommendations for the diagnosis of knee OA on the basis of research evidence and expert consensus.9 The EULAR group concluded that in persons older than 40 years who have use-related knee pain, a clinical diagnosis of OA may be made on the basis of 3 key symptoms-persistent knee pain, morning stiffness of only brief duration, and reduced function-and 3 findings on physical examination-crepitus, reduced movement, and bony enlargement.
The probability of the patient having knee OA was found to increase with an increasing number of positive features. For example, the probability was 19% if only knee pain was present, but it rose to 39% if limited morning stiffness and reduced function also were noted and to 99% when all 6 clinical features were present. For the purpose of making a diagnosis of knee OA, radiography-which is helpful in evaluating the severity of structural damage and is used widely in classification criteria and epidemiological studies-was considered an adjunct rather than a central component.
DIAGNOSTIC PITFALLS
Blint and Szebenyi10 published an excellent analysis of the problems that underlie the diagnosis of OA and the factors that confound clinicians in this area. The following sections draw extensively from their analysis.
Misinterpretation of the
patient's pain
Several common circumstances lead to misinterpretation of pain in patients with OA (Table 3). They include the following:
•The origin of the pain is not OA but some other type of arthritis, trauma, a neurological disorder, or soft tissue rheumatism that has occurred independent of the OA. Rheumatologic diseases are not mutually exclusive; patients with OA have no immunity from superimposed gout or staphylococcal infection of a joint with OA; a patient who has thumb base OA may coincidentally have de Quervain tenosynovitis.
•The pain is caused by OA but at a remote joint site. For example, pain in the knee often is referred from the hip. Radiculopathy resulting from OA of the apophyseal joints in the lumbar spine is a common cause of pain in the hip or gluteal region. Careful localization and characterization of the pain (Is it burning? Is it lancinating? Is numbness present?) can help the clinician make an accurate diagnosis.
•The pain is the result of soft tissue rheumatism that has developed secondary to the OA, such as anserine bursitis or collateral ligament strain in patients with knee OA.
Misinterpretation of deformity
In OA, deformity results from destruction of joint tissues, such as the articular cartilage and menisci; bony remodeling; osteophyte formation; and ligamentous damage. Deformities may be caused by diseases other than OA, some of which, like OA, may cause joint pain, gelling after periods of immobility, limitation of movement, crepitus, and bony swelling, leading to misdiagnosis. For example, hypertrophic pulmonary osteoarthropathy may be confused with nodal OA of the fingers, although clubbing of the nails and radiographic evidence of periostitis differentiate this condition from OA.
In patients with psoriatic arthritis of the DIP joints, the nail changes and skin lesions of psoriasis can establish the diagnosis. The skin lesions may be limited to the scalp line, perianal region, umbilicus, or external auditory canal and therefore require a careful and extensive examination of the integument for their detection.
In older persons, flexion contractures of joints, particularly of fingers, knees, and hips, may be attributed to OA when they are, in fact, the result of Dupuytren contracture, diabetic cheirarthropathy, trauma, or a neurological condition. Flexion contracture of the knee may be caused by a loose body; flexion contracture of the hip may be the result of osteonecrosis of the femoral head.
Neuropathic arthropathy (Charcot joint) can mimic OA. Characteristic features that differentiate this condition from OA are extensive periarticular ossification, ligamentous laxity, and severe deformity, with marked bony hypertrophy and, often, osteochondral fractures. Although neuropathic joint disease may be somewhat painless, it is not uncommon for a neuropathic joint to be severely painful and to exhibit acute signs of inflammation, raising concern about the presence of septic arthritis.
Careful neurological examination (with particular attention to impairment of position sense) is important in evaluating a patient in whom neuropathic arthropathy is suspected. Excluding acute bacterial joint infection may require arthrocentesis, with analysis of the synovial fluid, including a Gram stain and culture.
Misinterpretation
of the radiograph

Radiographs must be interpreted within the context of the patient's history and physical examination findings. Misinterpretation of radiographs is a common pitfall that leads to erroneous diagnosis in patients with OA (Table 4). For example:
•A patient with radiographic evidence of OA may present with history and physical examination findings that indicate a second type of arthritis, with no distinguishing radiographic characteristics at the time of presentation (eg, acute gout or pseudogout without radiographic evidence of a tophus or chondrocalcinosis, respectively, or acute bacterial joint infection before juxta-articular osteoporosis and destruction of the cortical margins have developed).
•Flexion of the knee caused by pain may result in joint-space narrowing on the conventional standing anteroposterior radiograph; this finding is misinterpreted as a loss of articular cartilage when, in fact, the joint is normal and the reduction in interbone distance is solely the result of the positioning of the joint.
•The patient may have diffuse idiopathic skeletal hyperostosis, with ossification of the anterior spinal ligament that is misinterpreted as vertebral osteophytosis.
•The patient may have a systemic metabolic abnormality, such as Wilson disease, hemochromatosis, or chondrocalcinosis, producing radiographic changes that are misinterpreted as garden-variety OA.
Misinterpretation
of laboratory results
Among the disorders that must be considered in the differential diagnosis for a patient with OA are
systemic inflammatory connective-tissue diseases, such as RA and systemic lupus erythematosus. Because OA principally is a disease of older persons, recognizing that the erythrocyte sedimentation rate (ESR) increases with age is important (Table 5); for example, an ESR of 50 mm/h, which would be abnormal for a person in his or her 20s or 30s, is consistent with age alone in someone in his 70s.
Because obesity is common in patients with OA (particularly knee OA), it is notable that a higher body mass index is associated with a higher serum C-reactive protein level.11 In addition, restricting the analysis to young adults (aged 17 to 39 years) and excluding cigarette smokers, patients who have clinically apparent inflammatory disease, cardiovascular disease, or diabetes mellitus and estrogen users did not change the results appreciably, suggesting the presence of low-grade systemic inflammation in obesity.
Titers of serum rheumatoid factor (RF) and antinuclear antibodies (ANAs) normally rise with age. Positive test results in older patients do not necessarily connote the presence of a systemic connective-tissue disorder. In most cases, a careful history and physical examination will differentiate these conditions from OA, obviating the need to obtain measurements of ESR, RF, and ANAs in older patients with OA.
References:
References1. Cushnaghan J, Dieppe P. Study of 500 patients with limb joint osteoarthritis, part I: analysis by age, sex, and distribution of symptomatic joint sites. Ann Rheum Dis. 1991;50:8-13.
2. Brown TD, Johnston RC, Saltzman CL, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739-744.
3. Földes K, Bálint P, Gaál M, et al. Nocturnal pain correlates with effusions in diseased hips. J Rheumatol. 1992;19:1756-1758.
4. Arnoldi CC, Linderholm H, Müssbichler H. Venous engorgement and intraosseous hypertension in osteoarthritis of the hip. J Bone Joint Surg. 1972;54B:409-421.
5. Radin EL. Osteoarthrosis-the ortopaedic surgeon's perspective. Acta Orthop Scand Suppl. 1995;266:6-9.
6. Massardo L, Watt I, Cushnaghan J, Dieppe P. Osteoarthritis of the knee joint: an eight year prospective study. Ann Rheum Dis. 1989;48:893-897.
7. Spector TD, Dacre JE, Harris PA, Huskisson EC. Radiological progression of osteoarthritis: an 11 year follow up study of the knee. Ann Rheum Dis. 1992;51:1107-1110.
8. Hernborg J, Nilsson BE. The relationship between osteophytes in the knee joint, osteoarthritis and aging. Acta Orthop Scand. 1973;44:69-74.
9. Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483-489.
10. Bálint G, Szebenyi B. Diagnosis of osteoarthritis: guidelines and current pitfalls. Drugs. 1996;52(suppl 3):S1-S13.
11. Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131-2135.
12. O'Reilly S, Doherty M. Signs, symptoms, and laboratory tests. In: Brandt KD, Doherty M, Lohmander SL, eds. Osteoarthritis. 2nd ed. Oxford, UK: Oxford University Press; 2003:197-210.