More aggressive treatment for juvenile idiopathic arthritis
ABSTRACT: Evidence of ongoing juvenile idiopathic arthritis (JIA) into adulthood has led to a shift in the treatment paradigm. Most physicians now pursue an approach of early, aggressive combination therapy.
As many as half of children who have juvenile idiopathic arthritis (JIA) enter adulthood with active disease,1 and evidence of ongoing disease into adulthood has led to a shift in the treatment paradigm. Rather than add medications gradually, most physicians now pursue an approach of early, aggressive combination therapy, including the use of NSAIDs; intra-articular corticosteroids; disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX); and biologic agents.
The treatment goal for JIA is to achieve disease remission to allow for normal childhood activities, growth, and development. Only a small percentage of children respond to NSAIDs alone. Radiological joint damage occurs in most patients with systemic disease or polyarthritis within 2 years and those with oligoarthritis within 5 years.2 Therefore, initial therapy should be vigorous to prevent long-term sequelae of destructive arthritis.
This 2-part article describes early diagnosis and management of JIA. In the first part (“Early identification of juvenile idiopathic arthritis,” The Journal of Musculoskeletal Medicine, February 2010, page 52), we reviewed the epidemiology, pathogenesis, clinical manifestations, classification, differential diagnosis, and laboratory analysis of JIA. This second part explores the treatment options.
APPROACHES TO TREATMENT NSAIDs
These are not disease-modifying medications, but they may be used to help control pain and diminish inflammation in all subtypes of JIA, particularly enthesitis-related arthritis (Table).2,3 NSAIDs that are FDA-approved for use in JIA include tolmetin, naproxen, meloxicam, and ibuprofen.2
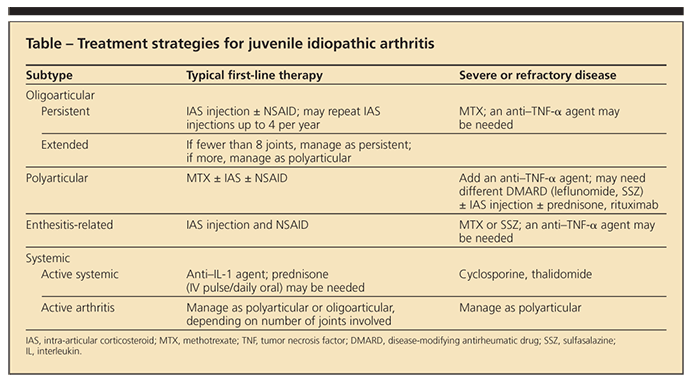
Adverse effects of NSAIDs include gastritis or duodenitis, cutaneous pseudoporphyria (particularly in children who have fair skin), and CNS effects (eg, headaches and behavior changes). Clinical trials in older adults have suggested an increased risk of cardiovascular events with NSAIDs, but no such studies have been conducted in children.2,4
Corticosteroids
Intra-articular corticosteroids are particularly useful for oligoarticular and psoriatic JIA and in joints resistant to medical therapy in other subtypes of JIA. Studies that used gadolinium contrast–enhanced MRI showed marked improvement of synovitis with no structural damage after intra-articular corticosteroid injection of 1 mg/kg of triamcinolone hexacetonide.3
Patients who had oligoarticular JIA and received intra-articular corticosteroids within the first 2 months of diagnosis demonstrated no leg length discrepancies compared with a group of children treated primarily with NSAIDs for several years.5 If a child requires more than 4 intra-articular corticosteroid injections per year, adding a DMARD or biologic agent is suggested.
Systemic corticosteroids are used in conjunction with a DMARD or biologic agent for rapid control of severe arthritis. They also are used in children who have systemic features of systemic JIA that do not respond to other interventions. Corticosteroids are weaned as rapidly as tolerated to avoid long-term adverse effects. Patients should not be treated with systemic corticosteroids alone.
Methotrexate
MTX is the DMARD of choice for children with JIA for whom NSAIDs or intra-articular corticosteroids are ineffective. Many pediatric rheumatologists start MTX at the first visit because it is the mainstay of treatment for most patients who have polyarticular disease.
MTX is a folic acid analogue that binds to dihydrofolate reductase, resulting in reduction of folates and leading to disruption of a variety of enzymatic pathways. A single weekly dose is effective in children (0.3 to 1 mg/kg or 10 to 30 mg/m2).4 Most patients who respond to MTX do so within 3 or 4 months.
Common adverse effects include elevation of aminotransferase levels, nausea, abdominal discomfort, and headache. Mucositis and alopecia are uncommon at the low doses of MTX used in JIA. GI adverse effects may improve if the medication is given subcutaneously rather than orally. Because folic acid depletion is thought to contribute to adverse effects, patients should take a multivitamin with folate.
Because there is a risk of hepatotoxicity, adolescents should be counseled against the use of alcohol with MTX. In addition, men and women should wait for 3 months after discontinuing MTX before trying to conceive because MTX is a powerful teratogen.2,4,6 Children should avoid live vaccines while taking MTX; the annual influenza vaccine is recommended. Patients should anticipate taking MTX for at least 1 year after remission.
Biologic agents
These agents are designed to target specific cytokines involved in JIA, such as tumor necrosis factor α (TNF-α), interleukin (IL)-1, and IL-6. Treatment results with these agents show promise but also raise concerns about risk of infection, responses to vaccinations, neurological adverse effects and long-term effects on immune surveillance, and risk of malignancy. The FDA recommends against combining biologic agents because of an increased incidence of infection. Adult screening guidelines for tuberculosis using purified protein derivative skin testing before biologic therapy is recommended.
Etanercept, a soluble TNF receptor antagonist, shows sustained benefits in most patients after 2 and 4 years.2 The agent is less effective in patients with systemic arthritis. Etanercept lowers the quantity of free TNF-α available for the maintenance of synovitis.3
The most common adverse effects of etanercept generally are mild; they include injection site reactions, upper respiratory tract infections, and headaches.7 Less common are neurological and psychiatric effects, severe infections, cutaneous vasculitis, and pancytopenia. Like MTX, etanercept is a weekly injection well tolerated by most patients.8
Uncontrolled studies show that the efficacy of infliximab is similar to that of etanercept. Infliximab is a monoclonal antibody based on a murine protein administered every 4 to 8 weeks intravenously. Children who have nongranulomatous uveitis have improved outcomes with infliximab use; this agent seems to be more effective than etanercept in controlling uveitis.3
Adalimumab is a TNF antagonist derived from human cells and is administered by subcutaneous injection weekly to every other week. A recent adalimumab trial showed a 78% response rate when the agent was given every other week. However, patients who received adalimumab in combination with MTX had a greater response rate than those taking adalimumab alone.3,8
In addition to anticipated therapeutic response, TNF antagonist selection may be influenced by adverse-effect profile and ease of administration. Some children have extreme anxiety with weekly injections and prefer monthly infusions. Of note, if a child does not respond to one of the TNF antagonists or effectiveness diminishes over time, it may be reasonable to try a second agent in the same class.
Other medical management
The host of disease-modifying and biologic agents that are used in children with JIA include leflunomide, cyclosporine, azathioprine, anakinra, rituximab, and abatacept; autologous stem cell transplant may be used. Each may have a place in the treatment of individual patients.
Surgical management
Orthopedic surgery has a limited role in the treatment of most children with JIA. However, surgical intervention may be necessary in cases of older children with persistent leg length discrepancy, joint contractures, or dislocations. Possible interventions to consider include arthroscopic synovectomy, soft tissue release, epiphysiodesis (growth plate fusion), total arthroplasty (joint replacement), and arthrodesis (joint fusion).4
Physical and occupational therapy
Such therapy maximizes musculoskeletal health and function in children who have chronic arthritis. Decreased physical activity, fitness, and function increase the risk of disability in children with JIA, increasing the risk of long-term cardiovascular and obesity-related morbidity. Regular exercise programs do not exacerbate arthritis and may even reduce disease symptoms.
SUMMARY
Once the diagnosis of JIA is established, treatment should be immediate and aggressive. A variety of therapies are available; the choice of treatment should be tailored to each JIA subtype. Early use of corticosteroid injections, MTX, and anti-TNF-α therapy has drastically improved outcomes.
References:
References1. Sullivan KE. Inflammation in juvenile idiopathic arthritis. Rheum Dis Clin North Am. 2007;33:365-388.
2. Hashkes PJ, Laxer RM. Medical treatment of juvenile idiopathic arthritis. JAMA. 2005;294:1671-1684.
3. Haines KA. Juvenile idiopathic arthritis: therapies in the 21st century. Bull NYU Hosp Jt Dis. 2007;65:205-211.
4. Cassidy JT, Petty RE. Chronic arthritis in childhood. In: Cassidy JT, Petty RE, Laxer RM, Lindsley CB, eds. Textbook of Pediatric Rheumatology. Philadelphia: Elsevier Saunders; 2005:206-260.
5. Sherry DD, Stein LD, Reed AM, et al. Prevention of leg length discrepancy in young children with pauciarticular juvenile rheumatoid arthritis by treatment with intraarticular steroids. Arthritis Rheum. 1999;42:2330-2334.
6. Wallace CA. Current management of juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol. 2006;20:279-300.
7. Gartlehner G, Hansen RA, Jonas BL, et al. Biologics for the treatment of juvenile idiopathic arthritis: a systematic review and critical analysis of the evidence. Clin Rheumatol. 2008;27:67-76.
8. Hayward K, Wallace CA. Recent developments in anti-rheumatic drugs in pediatrics: treatment of juvenile idiopathic arthritis. Arthritis Res Ther. 2009;11:216.