Occupational and Environmental Exposures in Lupus and Systemic Sclerosis
Diverse mechanisms of pathogenesis have been proposed for the autoimmune diseases. A growing body of medical literature suggests a potential effect of occupational exposures on their development.
The pathogenesis of systemic autoimmune diseases is a hotly contested topic given the uncertainty about the origins of these disorders. Throughout the years, diverse mechanisms of pathogenesis have been proposed, ranging from genetics and hormones to associations with various environmental triggering factors. What is well known is that most autoimmune diseases affect primarily women, suggesting a significant genetic or hormonal preference for this sex.
Frequent efforts have been made to link the development of disease to continuous exposure to toxins, chemicals, and radiation in various occupations, with various degrees of certainty. A growing body of medical literature suggests a potential effect of occupational exposures on the development of autoimmune diseases.
In this article, we review the existing epidemiological and experimental literature on occupational exposures and their correlation to the development of 2 autoimmune diseases that have significant morbidity: systemic sclerosis (SSc), a connective-tissue disorder of skin thickening, fibrotic organ damage, and vascular irregularities, and systemic lupus erythematosus (SLE), a chronic inflammatory systemic disease.
METHODS AND RESULTS
Cross-referencing the term “occupational exposure” with the terms “systemic sclerosis” and “systemic lupus erythematosus,” we searched MEDLINE (1966 - 2010) and PubMed (1966 - 2010) for available articles in the English language limited to humans. Retrieved articles were selected on the basis of their clinical relevance and applicability.
SSc and occupational
exposure
As early as 1914, case reports associating SSc with occupational and other environmental factors began to appear in the medical literature. A significant portion of the literature linking possible environmental and occupational exposures to SSc has come from case reports and small series and may lack scientific rigor.
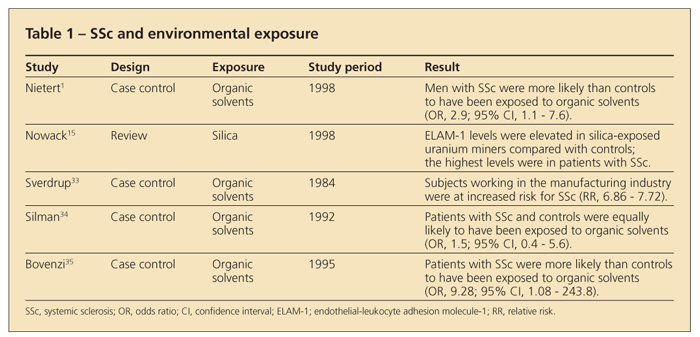
Although several studies have alluded to a possible association between SSc and certain genes, results from a modestly sized twin study indicate that 1 or more strong environmental factors probably are related to the disease pathogenesis (Table 1).1 Factors within case reports that might enhance confidence in a real link include the exposure having been massive in contrast to normal kinds of exposure; evidence that the person was not suitably protected and actually received substantial ingestion, by inhalation or other means; and the timing of the disease manifestations, with the pathology resulting from the exposure evolving into SSc.
A 2005 study investigated the potential association between occupational risk factors and severity markers of SSc in 105 patients.2 Exposures to silica dust, solvents, and epoxy resins were investigated in groups of 39 exposed patients and 66 unexposed ones. On the basis of the extent of cutaneous involvement, the disease was classified as limited or diffuse SSc. The immunological profile was determined by the presence of anti-topoisomerase I or anticentromere antibodies.
The study found that toxic products are associated with the more severe forms of SSc; mild forms of SSc were found in the unexposed patients. The severity markers were discovered to be associated with poor prognostic factors.3,4
The role of vinyl chloride
Vinyl chloride is the most well-reported organic solvent linked to an SSc-like illness. The first large study that proposed that long-term exposure to this substance is not harmless was conducted in 1963 in Romania, where 168 workers exposed to polyvinyl chloride were observed for 4 years.5 The workers reported pruritus of the arms and face and the development of SSc-like skin lesions that largely disappeared after they were removed from the workplace.
Similarly, occupational acro-osteolysis (OAOL)-the triad of sclerodermatous skin lesions, Raynaud phenomenon (RP), and osteolytic defects of the terminal phalanges-was first described in 2 autoclave cleaners in 1966.6 OAOL, found to occur only in persons exposed to the vinyl chloride monomer, is most common in reactor cleaners. The prevalence of OAOL in workers who had been in contact with the vinyl chloride monomer was assessed; a review of 5 studies from 4 countries showed 725 workers who were in contact with the monomer.
Acro-osteolysis developed in 3% of this group, RP developed in 10%, and sclerodermoid skin lesions developed in 6%. After cessation of the exposure, the skin lesions and the RP regressed more readily than the lytic lesions of the phalanges, which eventually healed.7
Even though it is similar to classic SSc, the SSc-like syndrome seen in vinyl chloride workers exhibits numerous features that help distinguish it. The cutaneous sclerosis in vinyl chloride workers affects predominantly the dorsum of the hands and the forearms, where the lesions may be papular, nodular, or plaque-like. There may be coarsening of face skin, particularly on the forehead and cheeks.7
In classic SSc, typically there is atrophy and tapering of the fingertips, with tufting of the distal phalanges. Other features of classic SSc not seen in vinyl chloride disease (VCD) include perioral puckering of the skin; subcutaneous calcification; and GI, renal, and cardiac involvement. Capillary microscopy abnormalities of the nail fold, such as dilated loops, microhemorrhages, and “drop out” areas, have been described in vinyl chloride workers; they are similar to but less extensive than those found in SSc.8
Vinyl chloride has been shown to produce highly reactive metabolites-chloroethylene oxide and chloroacetaldehyde-which have a high affinity for sulfhydryl groups in proteins and may result in covalent modification of these proteins, leading to self-recognition. Much of the concern about the risks of SSc related to chlorinated solvent exposures is based on structural similarities between vinyl chloride and chlorinated aliphatic solvents, such as trichloroethylene and perchloroethylene.9
Oxidation of intracellular thiols, via binding to these sulfhydryl groups, may lead to preferential inactivation of cytotoxic T lymphocytes. The loss of suppressor cell activity that results from this inactivation could lead to a breakdown in self-tolerance and contribute to the autoimmune response.10
However, in a case control study with population-based controls in Michigan and Ohio, dry cleaning did not show clear evidence of an association with SSc by self-report or after expert review.11 Most of the women who reported exposures to chlorinated hydrocarbon solvents did so in the dry cleaning industry. Dry cleaning typically involves the use of perchloroethylene and various chlorinated and nonchlorinated solvents that remove spots and stains at the service counter.11
Genetic factors also appear to be important in the pathogenesis of both SSc and the SSc-like syndrome seen in vinyl chloride workers. Black and associates7 compared HLA antigen frequencies and autoantibodies in patients with VCD, patients with SSc, asymptomatic workers, and normal controls. HLA-DR5 occurred more frequently in persons with VCD (36%) or SSc (30%) than in asymptomatic workers (3%) and normal controls (16%). In addition, more than half of the men with severe VCD had HLA markers B8 and DR3; none of the workers with mild disease were positive for these markers, suggesting that this haplotype favors progression of the disease.7
Silica exposure and SSc
Relationships between silica exposure and autoimmune disease have been observed.12-14 Endothelial-leukocyte adhesion molecule-1 (ELAM-1) and intercellular adhesion molecule-1 (ICAM-1) may be biomarkers of vascular injury in silica-exposed uranium miners who have SSc. ELAM-1 levels were elevated in silica-exposed uranium miners compared with those in age- and sex-matched population controls, and the highest levels occurred in miners with SSc.15 Up to 25% of miners with SSc showed strong elevations of this molecule in the serum compared with 11% of miners without symptoms.
ELAM-1 is expressed on vascular endothelial cells in response to cytokines (interleukin-1 [IL-1], tumor necrosis factor α [TNF-α]). Its level can be elevated in serum when these cells are strongly activated or destroyed, as occurs in several inflammatory and other disease conditions. However, ICAM-1 can be induced on dermal vascular endothelial cells by silica dust.
The association between silica exposure and the development of SSc is still unclear. Future studies may determine whether ELAM-1 and ICAM-1 act as disease mediators and may explore their use as biomarkers to determine the role of silica on vascular pathology in autoimmune diseases.
SLE and environmental
exposure
Environmental influences (eg, infectious agents and chemical compounds) may modulate an immune response on the development of SLE. Proposed mechanisms by which environmental exposures may influence SLE include production of autoreactive T cells and autoantibodies, stimulation of proinflammatory and anti-inflammatory cytokines, and targeting of end-organ damage. There have been numerous developments in lupus research pertaining to occupational silica exposure and autoimmune diseases since 1985. The number of epidemiological studies has increased substantially.
The role of silica in the
development of SLE
The biases that may arise in a single study of the association of silica and autoimmune disease may be overcome by using various types of study designs. The exposure assessment methods are critical. Occupational cohort studies of SLE are difficult to conduct given that SLE is a rare outcome, especially in men. Most patients with SLE being women presents a study challenge in that women are less likely than men to have career exposures to silica in the traditional trade industries that involve dust.
One way to determine the sensitivity of an exposure assessment method is to examine the sex-specific frequency of exposure among controls, which should be similar to that in studies in comparable populations. If the observed frequency of silica exposure is lower than expected, the assessment method probably will be an insensitive indicator of exposure.
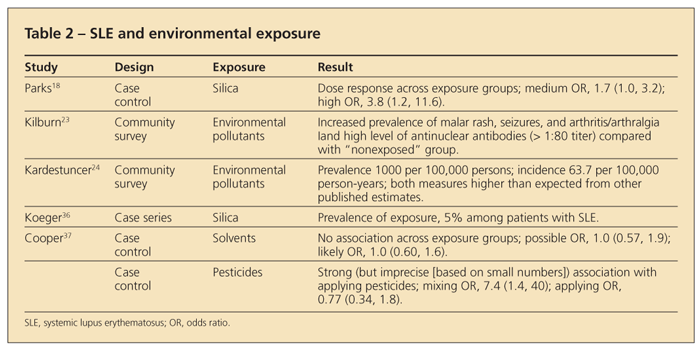
Six studies published since 1990 have provided data on occupational silica exposure and SLE. In a study in which 50 scouring powder factory workers were evaluated in Spain, 3 SLE cases and 5 SLE/SSc overlap cases were found.16 Similarly, Conrad and colleagues17 reported 28 definite and 15 possible SLE cases among 28,000 male uranium miners. Two other studies used various registries as data sources to examine SLE in patients with silicosis. A case-control study in the southeastern United States reported a dose-response association with silica dust exposure consistently seen across sex, racial, and educational subgroups (Table 2).18
Another consideration in evaluating the role of silica exposure in SLE is the relevant dose required for autoimmune effects. Evidence in previous studies of silica-related autoimmune disease suggests that exposure intensity may be a more important feature than cumulative life-time exposure levels.19 In addition, because silica is not metabolized or destroyed in the body, its effects may be quite long-lasting; therefore, exposure assessment should include experiences that occurred well before disease onset.
The adjuvant effects of silica, resulting in increased production of proinflammatory cytokines (TNF-α, IL-1), are well established in experimental animal studies. These effects are consistent with the observed pathological features of SLE (eg, inflammation, altered apoptosis). Silica is toxic to macrophages, which can lead to apoptosis and increased exposure to intracellular self-antigens.
Experimental studies that used the New Zealand mixed (NZM) lupus mouse strain demonstrated that silica exposure results in increased production of autoantibodies and immune complexes,20 as well as elevated levels of B1 a B cells and CD4+ T-cell counts and a higher ratio of regulatory T cells to helper T cells.21 Subsequent work revealed that the silica-induced exacerbation of autoimmunity in NZM mice is mediated by apoptosis and can be prevented by administration of rotterlin (an inhibitor of apoptosis). Experiments using animal models of silicosis have shown accumulation of silica in the lymph nodes, resulting in increased systemic immunoglobulin levels and elevated lymphocyte-derived interferon γ, which can activate macrophages and may lead to long-lasting expression of inducible nitric oxide synthase and maintenance of chronic inflammation.22
Solvents, pesticides, and air pollutants: relationship to SLE
Two community-based studies examined lupus or lupus symptoms in relation to various forms of pollutants. Kilburn and Warshaw23 reported contamination of the water supply with trichloroethylene and other solvents as well as heavy metals in Tucson and a higher prevalence of antinuclear antibodies and self-reported symptoms related to SLE in Tucson than in a “control” group in Phoenix. Kardestuncer and Frumkin,24 reporting a high prevalence of SLE in a small African American community in Georgia, hypothesized that environmental pollutants from industrial sources contributes to the disease. These 2 studies provide hypotheses that need to be examined in more focused environmental epidemiological studies using newly developed methods to assess exposure to pollutants.
There are few epidemiological studies linking pesticide exposure to SLE. Blood levels of dichlorodiphenyldichloroethylene, the long-lasting breakdown product of the organochlorine pesticide dichlorodiphenyltrichloroethane (DDT), and several organophosphate pesticide metabolites were examined in a small case-control study in Arizona.25 There was little difference in the distribution of these measurements between cases and controls. In fact, no association was seen with the more common activity of pesticide application (11% of cases and 15% of controls).
In contrast to the findings in human studies, several experimental studies in MRL+/+ mice have shown immune-related effects with exposure to trichloroethylene or some of its metabolites in drinking water or by intraperitoneal injection.22,26 These effects include increased production of autoantibodies, immunoglobulin, and interferon γ and activation of CD4+ T cells.
In 2008, a study explored the effect of lifetime farm and occupational organic dust exposures in patients with a recent diagnosis of SLE compared with controls.27 The results suggested an inverse association between SLE and agricultural exposure to animals, especially after extended exposure during childhood continuing into adult life. Notably, a reduced risk of atopy and allergic asthma had previously been associated with childhood agricultural exposures, especially to animals, lending support to the idea that early-life exposure to farm animals also may protect against the development of SLE.
The observation of an inverse association with SLE for adult farm contact with grains or corn was somewhat unexpected, based on previous literature suggesting that grain dust may be proinflammatory in agricultural workers.27 Therefore, it was suggested that in some settings, endotoxin or other organic dust exposure might protect against the development of SLE, although more studies would be needed to confirm such a finding.
Ultraviolet radiation
(UVR) exposure
UVR is hazardous to patients with SLE, and they are advised to minimize their exposure. Classically, this is accomplished through careful avoidance of sun exposure (using sunscreen, wearing long-sleeved clothing and hats) and strict avoidance of tanning booths.28
UVR exposure can induce and exacerbate skin lesions in patients with some subtypes of cutaneous lupus erythematosus. It may induce apoptosis of keratinocytes, whereas in SLE there is a defective clearance of apoptotic bodies, and it may be seen in overexpression of proinflammatory molecules, such as cytokines and chemokines, inducible nitric oxide synthase, and cellular adhesion molecules.29
Post-apoptotic debris accumulates in germinal centers, activates complement, and serves as a survival signal for B cells that had stochastically become autoreactive in the process of somatic hypermutation. In the presence of autoantibodies against apoptotic cells or adaptor molecules, the accumulation of post-apoptotic remnants (secondarily necrotic cell–derived material) causes immune complex formation and their pathological elimination, maintaining auto-inflammation.30
Apoptotic and necrotic keratinocytes not only may serve as a target for autoantibodies but also may release material (RNA and DNA) that induces the production of interferon α in plasmacytoid dendritic cells.31 This probably occurs through the formation of soluble immune complexes or via binding of IgG to apoptotic bodies or cells.32
DISCUSSION
The somewhat recent massive industrialization of society has resulted in increased concerns about environmental and, particularly, occupational exposures, including silica and asbestos in mining and vinyl chloride in manufacturing and construction. These exposures may have a substantial effect on the changing incidence of autoimmune diseases seen in the modern era.
A growing number of voices in the scientific community are suggesting the need for expanded research on the effects of modifiable occupational and environmental exposures on the pathogenesis of SLE, SSc, and other systemic autoimmune diseases. Given that the origins of the majority of autoimmune diseases remain unclear, better understanding of the exposure-disease relationship and its influence on the progression of the disease process would help create preventive measures and effective public health policies.
The use of comparable questionnaires and exposure evaluation methods across studies conducted in various populations would provide opportunities for both qualitative comparisons and more formal compilations of data, such as meta-analyses. Having larger, combined sample sizes along with harmonized exposure assessment methods also would allow for analyses of gene-environment interactions and better understanding of the role of occupational exposures in SLE.22
Both measuring exposure intensity and clarifying the timing of exposure are crucial in linking exposure to the disease pathogenesis. In addition, more research needs to be performed on the systemic immunogenic effects of harmful occupation-related substances and the autoimmune-related effects of varying exposure scenarios. Such research may directly affect the development of effective prevention strategies, because efforts to reduce cumulative exposures may require measures different from those in efforts to reduce short-term, high-intensity exposures.
Most of the epidemiological literature consists of exposure-disease relationships that have not been confirmed yet in experimental models. The lack of experimental data, where the effect of a single, specific agent in isolation from other compounds is carefully examined, makes it difficult to generalize the data from these uncontrolled studies.
Improved exposure assessment methods and better coordination between experimental models and epidemiological studies are needed to identify the risks more precisely. Global collaboration may be the industrialized nations’ best chance to clearly identify harmful occupational exposure and proactively protect workers and help regulate and safeguard the workplace.
References:
References
1. Nietert PJ, Sutherland SE, Silver RM, et al. Is occupational organic solvent exposure a risk factor for scleroderma? [published correction appears in Arthritis Rheum. 1998;41:1512]. Arthritis Rheum. 1998;41:1111-1118.
2. Magnant J, de Monte M, Guilmot JL, et al. Relationship between occupational risk factors and severity markers of systemic sclerosis. J Rheumatol. 2005;32:1713-1718.
3. Altman RD, Medsger TA Jr, Bloch DA, Michel BA. Predictors of survival in systemic sclerosis (scleroderma). Arthritis Rheum. 1991;34:403-413.
4. Medsger TA Jr, Silman AJ, Steen VD, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26:2159-2167.
5. Suciu I, Drejman I, Valaskai M. Contribution to the study of diseases caused by vinyl chloride. [in Romanian]. Med Interna (Bucur). 1963;15:967-978.
6. Harris DK, Adams WG. Acro-osteolysis occurring in men engaged in the polymerization of vinyl chloride. Br Med J. 1967;3:712-714.
7. Black C, Pereira S, McWhirter A, et al. Genetic susceptibility to scleroderma-like syndrome in symptomatic and asymptomatic workers exposed to vinyl chloride. J Rheumatol. 1986;13:1059-1062.
8. Veltman G, Lange CE, Jühe S, et al. Clinical manifestations and course of vinyl chloride disease. Ann N Y Acad Sci. 1975;246:6-17.
9. Brasington RD Jr, Thorpe-Swenson AJ. Systemic sclerosis associated with cutaneous exposure to solvent: case report and review of the literature. Arthritis Rheum. 1991;34:631-633.
10. Chiang SY, Swenberg JA, Weisman WH, Skopek TR. Mutagenicity of vinyl chloride and its reactive metabolites, chloroethylene oxide and chloroacetaldehyde, in a metabolically competent human B-lymphoblastoid line. Carcinogenesis. 1997;18:31-36.
11. Garabrant DH, Lacey JV Jr, Laing TJ, et al. Scleroderma and solvent exposure among women. Am J Epidemiol. 2003;157:493-500.
12. Berry G, Rogers A, Yeung P. Silicosis and lung cancer: a mortality study of compensated men with silicosis in New South Wales, Australia. Occup Med (Lond). 2004;54:387-394.
13. Steenland K. One agent, many diseases: exposure-response data and comparative risks of different outcomes following silica exposure. Am J Ind Med. 2005;48:16-23.
14. Bolton WK, Suratt PM, Strugill BC. Rapidly progressive silicon nephropathy. Am J Med. 1981;71:823-828.
15. Nowack R, Flores-Suárez LF, van der Woude FJ. New developments in pathogenesis of systemic vasculitis. Curr Opin Rheumatol. 1998;10:3-11.
16. Sanchez-Roman J, Wichmann I, Salaberri J, et al. Multiple clinical and biological autoimmune manifestations in 50 workers after occupational exposure to silica. Ann Rheum Dis. 1993;52:534-538.
17. Conrad K, Mehlhorn J, Lüthke K, et al. Systemic lupus erythematosus after heavy exposure to quartz dust in uranium mines: clinical and serological characteristics. Lupus. 1996;5:62-69.
18. Parks CG, Cooper GS, Nylander-French LA, et al. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: a population-based, case-control study in the southeastern United States. [published correction appears in Arthritis Rheum. 2004;50:1694]. Arthritis Rheum. 2002;46:1840-1850.
19. Aryal BK, Khuder SA, Schaub EA. Meta-analysis of systemic sclerosis and exposure to solvents. Am J Ind Med. 2001;40:271-274.
20. Brown JM, Archer AJ, Pfau JC, Holian A. Silica accelerated systemic autoimmune disease in lupus-prone New Zealand mixed mice. Clin Exp Immunol. 2003;131:415-421.
21. Brown JM, Pfau JC, Holian A. Immunoglobulin and lymphocyte responses following silica exposure in New Zealand mixed mice. Inhal Toxicol. 2004;16:133-139.
22. Parks CG, Cooper GS. Occupational exposures and risk of systemic lupus erythematosus: a review of the evidence and exposure assessment methods in population- and clinic-based studies. Lupus. 2006;15:728-736.
23. Kilburn KH, Warshaw RH. Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ Res. 1992;57:1-9.
24. Kardestuncer T, Frumkin H. Systemic lupus erythematosus in relation to environmental pollution: an investigation in an African-American community in North Georgia. Arch Environ Health. 1997;52:85-90.
25. Balluz L, Philen R, Ortega L, et al. Investigation of systemic lupus erythematosus in Nogales, Arizona. Am J Epidemiol. 2001;154:1029-1036.
26. Blossom SJ, Pumford NR, Gilbert KM. Activation and attenuation of apoptosis of CD4+ T cells following in vivo exposure to two common environmental toxicants, trichloroacetaldehyde hydrate and trichloroacetic acid. J Autoimmun. 2004;23:211-220.
27. Parks CG, Cooper GS, Dooley MA, et al. Childhood agricultural and adult occupational exposures to organic dusts in a population-based case-control study of systemic lupus erythematosus. Lupus. 2008;17:711-719.
28. Klein RS, Werth VP, Dowdy JC, Sayre RM. Analysis of compact fluorescent lights for use by patients with photosensitive conditions. Photochem Photobiol. 2009;85:1004-1010.
29. Kuhn A, Ruland V, Bonsmann G. Photosensitivity, phototesting, and photoprotection in cutaneous lupus erythematosus. Lupus. 2010;19:1036-1046.
30. Muñoz LE, Lauber K, Schiller M, et al. The role of incomplete clearance of apoptotic cells in the etiology and pathogenesis of SLE [in German]. Z Rheumatol. 2010;69:152, 154-156.
31. Lovgren T, Eloranta ML, BÃ¥ve U, et al. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861-1872.
32. BÃ¥ve U, Magnusson M, Eloranta ML, et al. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296-3302.
33. Sverdrup B. Do workers in the manufacturing industry run an increased risk of getting scleroderma? Int J Dermatol. 1984;23:629.
34. Silman AJ, Jones S. What is the contribution of occupational environmental factors to the occurrence of scleroderma in men? Ann Rheum Dis. 1992;51:1322-1324.
35. Bovenzi M, Barbone F, Betta A, et al. Scleroderma and occupational exposure. Scand J Work Environ Health. 1995;21:289-292.
36. Koeger AC, Marre JP, Rozenberg S, et al. Autoimmune diseases after unusual exposure to silica or silicones: 3 cases [in French]. Ann Med Interne (Paris). 1992;143:165-170.
37. Cooper GS, Parks CG. Occupational and environmental exposures as risk factors for systemic lupus erythematosus. Curr Rheumatol Rep. 2004;6:367-374.